Moles
Name:
Chemistry Chapter 12 Study Guide
12.1
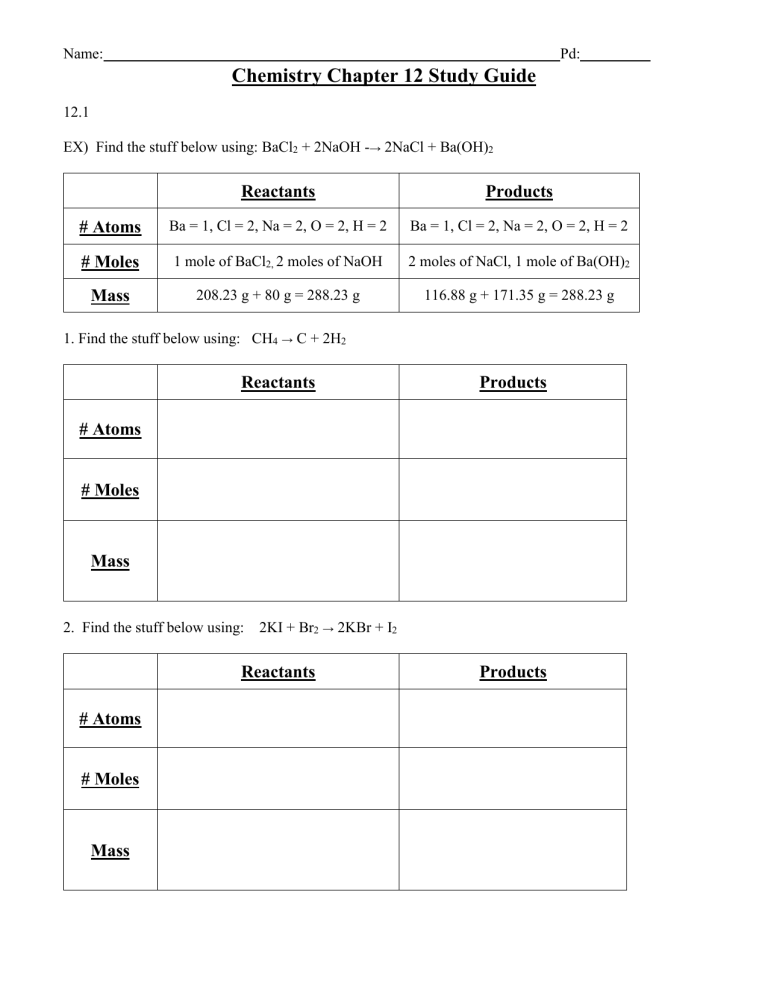
EX) Find the stuff below using: BaCl
2
+ 2NaOH → 2NaCl + Ba(OH)
2
Reactants Products
Pd:
# Atoms Ba = 1, Cl = 2, Na = 2, O = 2, H = 2 Ba = 1, Cl = 2, Na = 2, O = 2, H = 2
# Moles 1 mole of BaCl
2,
2 moles of NaOH 2 moles of NaCl, 1 mole of Ba(OH)
2
Mass 208.23 g + 80 g = 288.23 g
1. Find the stuff below using: CH
4
→ C + 2H
2
Reactants
116.88 g + 171.35 g = 288.23 g
Products
# Atoms
# Moles
Mass
2. Find the stuff below using: 2KI + Br
2
→ 2KBr + I
2
Reactants
# Atoms
# Moles
Mass
Products
Name: Pd:
12.2
3. Convert 6.3 mol of Cu into mol of AgNO
3.
2 AgNO
3
+ Cu
Cu(NO
3
)
2
+ 2 Ag
4. If I want 5.67 mols of F
2
, how many grams of CF
4
do I need to start with?
CF
4
+ 2 Br
2
CBr
4
+ 2 F
2
5. How many mols of Cs will be produced by using 9.5 L of GaF
3
?
GaF
3
+ 3 Cs
3 CsF + Ga
6. How many liters of NH
3
at STP will be produced from 7.3e24 molecules of hydrogen?
N
2
+ 3 H
2
2 NH
3
7. How many grams of NaF will be produced when reacted with 55.1 g of F
2
?
2 NaF + Br
2
2 NaBr + F
2
8. What mass of KBr will form with 5.5e24 formula units of Al
2
(SO
4
)
3
?
2 AlBr
3
+ 3 K
2
SO
4
6 KBr + Al
2
(SO
4
)
3
Name: Pd:
12.3 – Instructions Recap
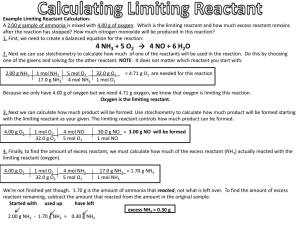
EX) A. What is the limiting reactant when forming PH
3 using 22.1 g of P reacts and 40.1 g of H
2
?
Equation: 2 P + 3 H
2
2PH
3
Step 1. Do two conversions, converting the mass of each reactant (P and H
2
) into grams of product (PH
3
.)
The limiting reactant is the reactant that gives the SMALLEST amount of product.
-After doing the conversions, 22.1 g of P gets 24.3 g of PH
3
, and 40.1 g of H
2
gets 450 g of PH
3
- The limiting reactant is P
B. How many grams of PH
3
are formed?
Step 2. See how many grams of product were formed from the limiting reactant : 24.3 g of PH
3
C. Calculate how many grams of excess reagent are needed to react with the limiting reactant.
Step 3. Do another conversion, converting the grams of limiting reactant into grams of the other reactant .
-Converting 22.1 g of P into grams of H
2
gets us 2.1 g of H
2
D. How much excess reagent is leftover after the reaction is complete?
Step 4. Subtract the amount given in the problem from the amount you just calculated
40.1 g H
2
- 2.1 g of H
2
= 38.0 g of excess H
2
E. If the actual yield is 15.5 g of PH
3
, what is my percent yield?
Step 5. (Actual / Theoretical) * 100 = (15.5 g / 24.3 g ) * 100 = 63.8%
Name: Pd:
9. 2. A. When looking at copper, what is the limiting reactant when 78.8 g of Al reacts with 99.3 g of
CuS?
Equation: 2 Al + 3 CuS
3Cu + Al
2
S
3
B. How many grams of Cu are formed?
C. Calculate how many grams of excess reagent are needed to react with the limiting reactant.
D. How much excess reagent is leftover after the reaction is complete?
E. If I actually got 78.9 g Cu in my experiment, what is my percent yield?
Name: Pd:
10. A. What is the limiting reactant when forming NH
3
with 21.1 g of N
2
and 15.4 g of H
2
?
Equation: N
2
+ 3 H
2
2 NH
3
B. How many grams of NH
3
are formed?
C. Calculate how many grams of excess reagent are needed to react with the limiting reactant.
D. How much excess reagent is leftover after the reaction is complete?
E. If I actually got 876 g NH
3
in my experiment, what is my percent yield?