Proteins and Enzymes JIT
advertisement
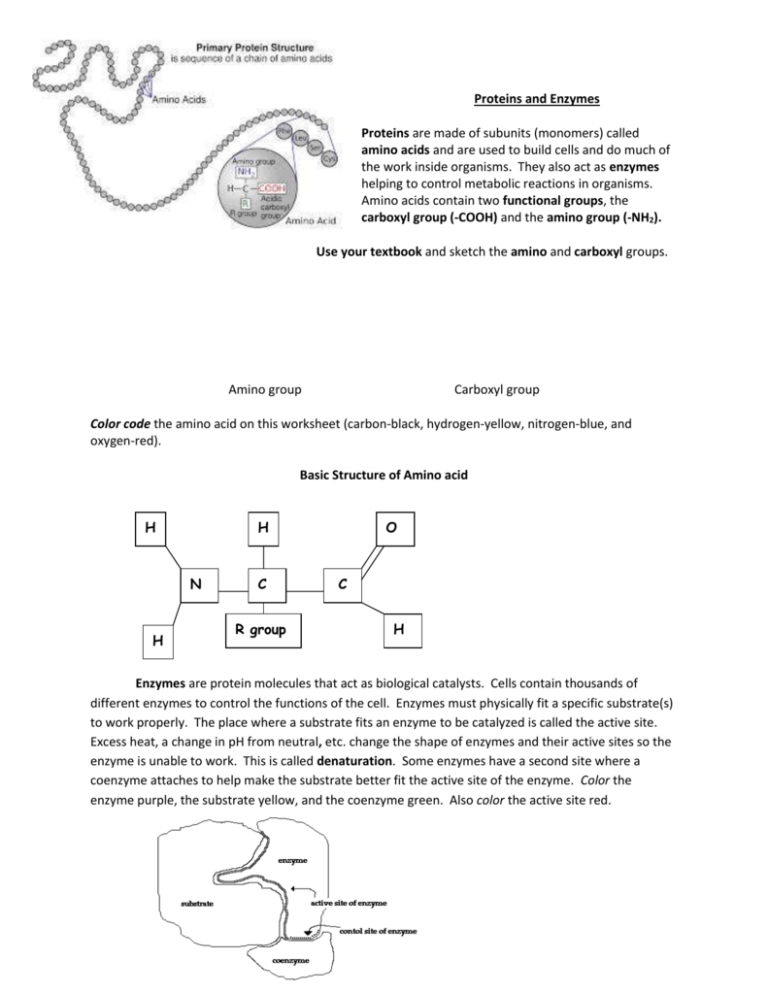
Proteins and Enzymes Proteins are made of subunits (monomers) called amino acids and are used to build cells and do much of the work inside organisms. They also act as enzymes helping to control metabolic reactions in organisms. Amino acids contain two functional groups, the carboxyl group (-COOH) and the amino group (-NH2). Use your textbook and sketch the amino and carboxyl groups. Amino group Carboxyl group Color code the amino acid on this worksheet (carbon-black, hydrogen-yellow, nitrogen-blue, and oxygen-red). Basic Structure of Amino acid H H N H C R group O C H Enzymes are protein molecules that act as biological catalysts. Cells contain thousands of different enzymes to control the functions of the cell. Enzymes must physically fit a specific substrate(s) to work properly. The place where a substrate fits an enzyme to be catalyzed is called the active site. Excess heat, a change in pH from neutral, etc. change the shape of enzymes and their active sites so the enzyme is unable to work. This is called denaturation. Some enzymes have a second site where a coenzyme attaches to help make the substrate better fit the active site of the enzyme. Color the enzyme purple, the substrate yellow, and the coenzyme green. Also color the active site red. Enzymes and the Environment Sometimes we like things just right. If it's too hot, we swelter under the sun. If it's too cold, we shiver. If there's too much to eat, we hit a point where we can't swallow another bite. But when we're hungry, there never seems to be enough food in the house. It's not that we're nitpicky, right? It's just that we're human, and, after all, we know what we like and we know what conditions suit us best. We are not the only ones who value the perfect conditions. Plenty of animals and other organisms do, too. And on a microscopic level, so do our cells. Even within our cells, enzymes, the proteins that catalyze chemical reactions, like things just right, too. As a quick review, an enzyme works on a substrate, or substance or molecule on which an enzyme functions. An active site is where a substrate binds an enzyme in order to facilitate a reaction. Each enzyme works on a specific substrate in a specific condition. These conditions are optimal for the enzyme - just like how the perfect amount of sunshine and food keep us satisfied and happy. Why do we need enzymes?? One of the immutable laws of nature is that energy is required to initiate a chemical reaction. After all, if you want to change something, you have to put some work into it. For every chemical reaction, a specific amount of energy - the energy of activation must be applied to the reagents before the reaction will proceed. The energy of activation is like a hill between the reagents and the product, and the reagents must be pushed over this hill before the reaction can continue. For some reactions, the energy of activation is so high that your cells either cannot supply it or would be harmed by the release of heat as the reaction proceeded. In other cases, a given reaction might proceed at a pace that is normally too slow to meet the cell's metabolic demands. That's where enzymes come in: they lower the energy 'hill' so chemical reactions can get started more easily and proceed in conditions that are 'cell friendly.' Enzymes along a metabolic pathway can also divide one high-energy chemical reaction into many steps, each requiring only a fraction of the energy needed for the whole process. Finally, enzymes can accelerate the rate of a reaction millions of times over, which is critical to cells that often need large quantities of a particular product within just a few seconds' time. Effect of Temperature Just like it affects us, temperature also affects enzyme function. Enzymes work optimally at a specific temperature. Again, different enzymes might have different optimum temperatures. Colder weather slows much of life down, and cold temperatures will also slow the reaction rate of enzymes. When things heat up, molecules move faster, similar to how we go outside or run around when the weather starts to warm up in the spring. An increase in temperature usually means more movement and a greater likelihood that molecules collide. Higher temperatures can increase the rate of reaction between an enzyme and its substrate, but only to a point. This temperature point of maximum function is called an enzyme's optimum temperature. As you can probably guess, the optimum temperature for enzymes in our body is actually our body's temperature! If the temperature gets too high, it can distort the enzyme, making it unable to function properly. In fact, if temperatures got too high, enzymes can become denatured, or undergo a structural change in shape that inhibits function. If the change in shape affects the active site, then an enzyme may no longer recognize a substrate. With more enzymes becoming more non-functional, the rate of the reaction decreases with an increase in temperature. Effect of pH Enzymes are affected by changes in pH. The most favorable pH value - the point where the enzyme is most active - is known as the optimum pH. This is graphically illustrated in Figure 14. Extremely high or low pH values generally result in complete loss of activity for most enzymes. pH is also a factor in the stability of enzymes. As with activity, for each enzyme there is also a region of pH optimal stability. The optimum pH value will vary greatly from one enzyme to another. In addition to temperature and pH there are other factors, such as ionic strength, which can affect the enzymatic reaction. Each of these physical and chemical parameters must be considered and optimized in order for an enzymatic reaction to be accurate and reproducible.