rDNA Registration - Office of Research Integrity Assurance
advertisement

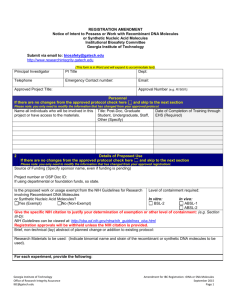
REGISTRATION Notice of Intent to Possess or Work with Recombinant DNA Molecules or Synthetic Nucleic Acid Molecules Institutional Biosafety Committee Georgia Institute of Technology Office of Research Integrity Assurance Use Only File Number Date Received Submit via email to ibc@gatech.edu http://www.researchintegrity.gatech.edu (This form is in Word and will expand to accommodate text). Personnel Principal Investigator PI Title Telephone Fax Co-PI Co-PI Title Telephone Fax Department Email Department Email Lab contact person other than PI or Co-PI: Telephone Email Name all individuals who will be involved in this project or have access to the materials. Check category of this project: Project Title Title: Post-Doc, Graduate Student, Undergraduate, Staff, Other (Specify) Date of Completion of Training through EHS (Required) Title & Funding New Proposal Teaching/Training Research Source of Funding (Specify sponsor name, even if funding is pending). Project number or OSP Doc ID: If using departmental or foundation funds, so state. Details of Proposed Use Level of containment required: Is the proposed work or usage exempt from the NIH Guidelines for Research involving Recombinant DNA Molecules In vitro: In vivo: or Synthetic Nucleic Acid Molecules? BSL-2 ABSL-1 Yes (Exempt) No (Non-Exempt) ABSL-2 Give the specific NIH citation to justify your determination of exemption or other level of containment: (e.g. Section III-D): NIH Guidelines can be viewed at: http://oba.od.nih.gov/rdna/nih_guidelines_oba.html Registration approvals will be withheld unless the NIH citation is provided. Brief, non-technical (lay) abstract of planned work: Georgia Institute of Technology Office of Research Integrity Assurance IBC IBC@gatech.edu IBC Registration: rDNA or SNA Molecules September 2015 Page 1 Research Materials to be used: (Indicate binomial name and strain of the recombinant or synthetic DNA molecules to be used). For each experiment, provide the following: Source of the DNA (organisms): Nature of the inserted sequences: Vector Details: Please provide/attach Vector maps for each vector you will be using. Describe below the safety precautions for working with each vector, including whether it is self-inactivating, self-replicating, etc. List host cells, vectors, recombinant materials: (E. coli, K-12 system, Saccharomyces system, insect cells, plasmids, cosmids, phages, etc.). Please list any cell lines that will be used or modified (human or animal) providing the cell line and strain: Are any of the following genes, viruses, factors, or conditions involved? Mark Yes or No for each field below Deliberate transfer of drug resistance into organisms that do not acquire them naturally? (except for approved host-vector systems that contain antibiotic resistance markers) Deliberate transfer of Recombinant DNA or Synthetic Nucleic Acid Molecules into humans? Genes that produce vertebrate toxins with LD50 less than 100 ng/kg of body weight? Using human or animal pathogens as host-vector systems? Human or animal pathogen DNA cloned into non-pathogenic prokaryote or lower eukaryote? Using infectious animal or plant DNA or RNA viruses in tissue culture or using defective viruses when a helper is present? Altering an animal genome by Recombinant DNA or Synthetic Nucleic Acid Molecules or testing viable modified microorganisms in whole animals? Genetic engineering of plants by Recombinant DNA or Synthetic Nucleic Acid Molecules methods or use of plants with microorganisms or insects containing rDNA? Experiments involving more than 10 liters of culture? Deliberate release of Recombinant DNA or Synthetic Nucleic Acid Molecules modified plants or animals into the environment? Location Building Room Telephone Indicate where project will be conducted Georgia Institute of Technology Emory University Grady Hospital VA Medical Center Other (specify) Georgia Institute of Technology Office of Research Integrity Assurance IBC IBC@gatech.edu IBC Registration: rDNA or SNA Molecules September 2015 Page 2 Containment and Safety Equipment Will a biological safety cabinet (BSC) be used? Type of BSC: Class II A1 A2 B1 B2 Yes No Manufacturer name: Date of last certification: Will a horizontal or vertical laminar flow “clean bench” be used for the planned activity? Yes No Other safety equipment to be used: Method of decontamination of biological or infectious wastes: Safety pipettes Autoclave Centrifuge Building______________________ Room _______ Safety cups Incinerator Chemical fume hood Chemical Disinfectant (Specify) Note: Bleach and Autoclaving may never be used in Others: combination As a result of this project, will there be any ultratoxic, shock sensitive or other chemicals which will require special handling during use, storage or disposal? Yes No Describe in detail the method of disposal of chemical or biological wastes: Have the safety precautions included in the Material Safety Data Sheets been incorporated into your procedures? Yes No Other boards or committees that must review Submittal Protocol Status and approve this proposed use: date number Pending Approved Chemical & Environmental Safety Committee Institutional Review Board Institutional Animal Care & Use Committee Radiation Safety Biological Materials Safeguards Committee You may also need to coordinate with the Biosafety Officer, the Responsible Official, and/or Environmental Health and Safety. Dual Use Research of Concern (DURC) Potential Assessment Despite its value and benefits, certain types of research conducted for legitimate purposes can be utilized for both benevolent and harmful purposes. Such research is called Dual Use Research (DUR). Dual Use Research of Concern (DURC) is a subset of DUR and is defined as “life sciences research that, based on current understanding, can be reasonably anticipated to provide knowledge, information, products, or technologies that could be directly misapplied to pose a significant threat with broad potential consequences to public health and safety, agricultural crops and other plants, animals, the environment, materiel, or national security.” On March 29, 2012, the US Government released the US Government Policy for Oversight of Life Sciences Dual Use Research of Concern to establish the requirements for the oversight of DURC by the US Government. On September 24, 2014, the US Government Policy for Institutional Oversight of Life Sciences Dual Use Research of Concern was released to establish the requirements for institutional (i.e., non-US Government) oversight of DURC. The US Government considers these two policies to be complementary. These definitions could potentially encompass a number of life sciences research projects at Georgia Tech, however, the current scope of the Policy has been limited to the following agents and toxins and categories of experiments. Research must involve both a listed agent/toxin and category of experiment to be deemed potential DURC. Georgia Institute of Technology Office of Research Integrity Assurance IBC IBC@gatech.edu IBC Registration: rDNA or SNA Molecules September 2015 Page 3 Agent or Toxin Involved in Project (check all that apply) Verify if this project directly involves non-attenuated forms of 1 or more of the 15 listed agents. Avian Influenza (highly pathogenic) Bacillus anthracis Botulinum neurotoxin (any quantity) Burkholderia mallei Burkholderia pseudomallei Ebola virus Foot-and-mouth disease virus Francisella tularensis Marburg virus Reconstructed 1918 influenza virus Rinderpest virus Toxin producing strains of Clostridium botulinum Variola major virus Variola minor virus Yersinia pestis NONE Experimental Effects (check all that apply) Indicate whether the research project indicated above produces, aims or can be reasonably anticipated to produce any of the following experimental effects. Enhances the harmful consequences of the agent or toxin. Disrupts the immunity or the effectiveness of an immunization against the agent or toxin without clinical or agricultural justification. Confers to the agent or toxin resistance to clinically or agriculturally useful prophylactic or therapeutic interventions against the agent or toxin or facilitates its ability to evade detection methodologies. Alters properties of the agent or toxin in a manner that would enhance its ability to be disseminated. Alters the host range or tropism of the agent or toxin. Enhances the susceptibility of a host population to the agent or toxin. Generates or reconstitutes an eradicated or extinct agent or toxin listed in Question 6.2 of this form. NONE If you checked any of the above experimental effects, please explain: Previous Work Experience and Additional Information What is the research team’s previous work experience with the agent/material(s) specified in this application? Please indicate any additional information or comments pertinent to the Institutional Biosafety Committee review: Biological Hygiene Plan for Biosafety Registrations Involving rDNA A current Biological Safety Plan for rDNA or Synthetic Nucleic Acid Registrations must be submitted for all exempt and non-exempt registrations. The template is available at http://researchintegrity.gatech.edu/about-ibc/ibc-registration-forms. Certifications and Signatures PI CERTIFICATION: I have read and am familiar with the standard and special microbiological practices, containment equipment, personal protective equipment, and laboratory facilities recommended for the biosafety level applicable to this project. I will ensure that all faculty, staff, and students working on this project will follow these recommendations as a condition of Institutional Biosafety Committee approval of this project. I am submitting the following required documents for review by the Institutional Biosafety Committee: IBC Registration (this form) Biological Safety Plan (sample at http://researchintegrity.gatech.edu/about-ibc/ibc-registration-forms) Grant pages or technical abstract for this project, if any Date: Principal Investigator Signature _____________________(or submit via GIT email for electronic signature) Georgia Institute of Technology Office of Research Integrity Assurance IBC IBC@gatech.edu IBC Registration: rDNA or SNA Molecules September 2015 Page 4 Department Chair/GTRI Laboratory Director Signature: I have reviewed this DNA registration and related research plans and concur with their submission to the Institutional Biosafety Committee for review. Date: Signature _____________________(or submit via GIT email for electronic signature) In lieu of dept head/lab director signatures, the Office of Research Integrity Assurance will accept an email from the dept head/lab director, sent to biosafety@gatech.edu, in which he/she states something like: “I have reviewed this registration and related research plans and concur with their submission to the Institutional Biosafety Committee for review.” Georgia Institute of Technology Office of Research Integrity Assurance IBC IBC@gatech.edu IBC Registration: rDNA or SNA Molecules September 2015 Page 5