jcpe12507-sup-0018-AppendixS1
advertisement

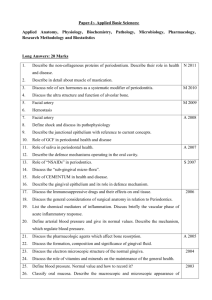
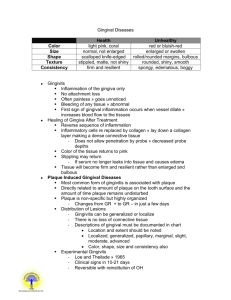
APPENDIX SUPPLEMENTARY METHODS Clinical examinations and sample collection Clinical examinations and diagnosis were performed according to the criteria of the American Academy of Periodontology (Armitage, 2004). In addition to baseline readings, clinical examinations using a calibrated periodontal probe (UNC 15; Hu-Friedy) were performed at 1, 2, 4, and 6 weeks during the treatment period and continued throughout the follow-up period at 7, 8, 10, and 12 weeks. The examinations included determination of probing pocket depth (by measuring the distance [mm] from the gingival margin to the base of the pocket), clinical attachment level (distance from the cementoenamel junction to the base of the pocket), gingival index (using a scale of 0-3, according to Loe (Loe, 1967)), bleeding on probing (% positive sites), plaque index (scale of 0-3 according to Loe (Loe, 1967)), and tooth mobility index (scale 0-3, using a modification of the Miller index according to Laster et al (Laster et al., 1975)). The determination of probing pocket depth, clinical attachment level, and bleeding on probing was performed at six sites, specifically at the mesio-buccal, mid-buccal, disto-buccal, mesio-lingual, mid-lingual and disto-lingual aspects of each of the anterior and posterior teeth. Gingival index and plaque index were assessed at four sites, specifically, at the mesio-buccal, mid-buccal, disto-buccal and midlingual aspects of each of the anterior and posterior teeth. At baseline, 1, 2, 4, 6, 9 and 12 weeks, the teeth were dried and isolated with cotton rolls and GCF was collected using methylcellulose strips (PerioPaper; Oraflow) placed between the gums and the teeth, 1 specifically in the mesio-buccal sulcus, until mild resistance was felt and kept in place for 60 s. GCF was collected from two teeth (from each jaw) that exhibited the deepest pockets at baseline, and the same teeth were sampled throughout the study. At each time point, the two GCF samples per jaw of each monkey were assayed independently, and the data were averaged for use in the statistical analysis (n = 5 animals for “1x-treatment”; n = 10 animals for “3x-treatment”). At baseline, 6 weeks, and at the completion of the study (12 weeks), biopsies of gingiva and bone were removed en bloc corresponding to first or second molars that exhibited the deepest pockets at baseline. At each time point, two biopsies (one per jaw) were taken from each animal. During the procedures, the animals were anesthetized with an intramuscular injection of 5-10 mg/kg ketamine and 1-2 mg/kg xylazine. Anesthesia was monitored by checking palpebral reflex, toe pinch and blood oxygen level (SpO2). Immunofluorescence histochemistry Gingival biopsy specimens were fixed in 4% paraformaldehyde and embedded in OCT compound. Mesio-distal sections were stained using the following primary antibodies (all from Abcam): Rabbit polyclonal antibody to IL-17A, rabbit monoclonal antibody to C3d (clone E28-P) or mouse monoclonal antibodies to receptor activator of nuclear factor-κB ligand (RANKL) (clone 12A668), osteoprotegerin (OPG) (clone 5G2), C5a/C5a-desArg (clone 2942), or C3a/C3a-desArg (clone 4H3). Secondary reagents included AlexaFluor488– or AlexaFluor594–conjugated goat anti-rabbit IgG, or AlexaFluor594– conjugated goat anti-mouse IgG (Life technologies). The specificity of staining was confirmed by using appropriate isotype controls or non-immune rabbit IgG followed by 2 AlexaFluor488– or AlexaFluor594–conjugated anti-IgG. Images were captured using a Nikon Eclipse NiE automated upright fluorescent microscope. Histological TRAP staining Bone biopsy specimens from the maxillae or mandibles of monkeys were fixed in 4% paraformaldehyde, decalcified in Immunocal solution (StatLab) for 14 d followed by placement in Cal-Arrest solution (StatLab) to neutralize the pH of the tissue, and then embedded in OCT compound. TRAP staining was performed on mesio-distal sections (6- to 8-μm thick) using the Tartrate-Resistant Acid Phosphatase kit (Clontech). Slides were viewed using a Nikon Eclipse Ni-E microscope. TRAP-positive multinucleated cells were considered to be osteoclasts. Host immune responses Cytokine and immune mediator protein levels in GCF samples eluted as previously described (Bostanci et al., 2007) were measured using Milliplex xMap kits (for IL-1β, IL-6, IL-8, IL-17, RANKL and OPG; Millipore) or bead-based multiplex assays (only for C5/C5a; R&D Systems) on a Bio-Plex system (Bio-Rad). The GCF levels of C3a were assayed by ELISA using a kit from eBioscience. Gingival tissue from biopsies was used to extract total RNA (using Trizol; Life Technologies), which was quantified by spectrophotometry at 260 and 280 nm. The RNA was reverse-transcribed using the High Capacity RNA-to-cDNA Kit (Life Technologies) and real-time PCR with cDNA was performed using the Applied Biosystems 7500 Fast Real-Time PCR System according to the manufacturer's protocol (Life Technologies). Data were analyzed using the comparative (ΔΔCt) method. TaqMan 3 probes, sense primers, and antisense primers for detection and quantification of genes investigated in this paper were purchased from Life Technologies. The primers were specific for human genes and cross-reacted with the reported non-human primate genes (Fig. 6). 4 SUPPLEMENTARY FIGURES LEGENDS: Figure S1. Effects of Cp40 on inflammatory clinical parameters of NHP chronic periodontitis (3X treatment): Raw data for monkey #1. Cp40 was injected – three times weekly for 6 weeks – into the interdental papillae and the distal gingiva of the second molars of the maxilla (“Cp40”), whereas the mandible was not treated (“Untreated”). The animal was clinically examined at the indicated time points and the following clinical parameters were recorded: (A) gingival index; (B) bleeding on probing; (C) probing pocket depth; (D) clinical attachment level; (E) mobility index; and (F) plaque index. Figure S2. Effects of Cp40 on inflammatory clinical parameters of NHP chronic periodontitis (3X treatment): Raw data for monkey #2. Cp40 was injected – three times weekly for 6 weeks – into the interdental papillae and the distal gingiva of the second molars of the maxilla (“Cp40”), whereas the mandible was not treated (“Untreated”). The animal was clinically examined at the indicated time points and the following clinical parameters were recorded: (A) gingival index; (B) bleeding on probing; (C) probing pocket depth; (D) clinical attachment level; (E) mobility index; and (F) plaque index. Figure S3. Effects of Cp40 on inflammatory clinical parameters of NHP chronic periodontitis (3X treatment): Raw data for monkey #3. Cp40 was injected – three times weekly for 6 weeks – into the interdental papillae and the distal gingiva of the second molars of the maxilla (“Cp40”), whereas the mandible was not treated (“Untreated”). The animal was clinically examined at the indicated time points and the following clinical 5 parameters were recorded: (A) gingival index; (B) bleeding on probing; (C) probing pocket depth; (D) clinical attachment level; (E) mobility index; and (F) plaque index. Figure S4. Effects of Cp40 on inflammatory clinical parameters of NHP chronic periodontitis (3X treatment): Raw data for monkey #4. Cp40 was injected – three times weekly for 6 weeks – into the interdental papillae and the distal gingiva of the second molars of the maxilla (“Cp40”), whereas the mandible was not treated (“Untreated”). The animal was clinically examined at the indicated time points and the following clinical parameters were recorded: (A) gingival index; (B) bleeding on probing; (C) probing pocket depth; (D) clinical attachment level; (E) mobility index; and (F) plaque index. Figure S5. Effects of Cp40 on inflammatory clinical parameters of NHP chronic periodontitis (3X treatment): Raw data for monkey #5. Cp40 was injected – three times weekly for 6 weeks – into the interdental papillae and the distal gingiva of the second molars of the maxilla (“Cp40”), whereas the mandible was not treated (“Untreated”). The animal was clinically examined at the indicated time points and the following clinical parameters were recorded: (A) gingival index; (B) bleeding on probing; (C) probing pocket depth; (D) clinical attachment level; (E) mobility index; and (F) plaque index. Figure S6. Effects of Cp40 on inflammatory clinical parameters of NHP chronic periodontitis (3X treatment): Raw data for monkey #6. Cp40 was injected – three times weekly for 6 weeks – into the interdental papillae and the distal gingiva of the second molars of the maxilla (“Cp40”), whereas the mandible was not treated (“Untreated”). The 6 animal was clinically examined at the indicated time points and the following clinical parameters were recorded: (A) gingival index; (B) bleeding on probing; (C) probing pocket depth; (D) clinical attachment level; (E) mobility index; and (F) plaque index. Figure S7. Effects of Cp40 on inflammatory clinical parameters of NHP chronic periodontitis (3X treatment): Raw data for monkey #7. Cp40 was injected – three times weekly for 6 weeks – into the interdental papillae and the distal gingiva of the second molars of the maxilla (“Cp40”), whereas the mandible was not treated (“Untreated”). The animal was clinically examined at the indicated time points and the following clinical parameters were recorded: (A) gingival index; (B) bleeding on probing; (C) probing pocket depth; (D) clinical attachment level; (E) mobility index; and (F) plaque index. Figure S8. Effects of Cp40 on inflammatory clinical parameters of NHP chronic periodontitis (3X treatment): Raw data for monkey #8. Cp40 was injected – three times weekly for 6 weeks – into the interdental papillae and the distal gingiva of the second molars of the maxilla (“Cp40”), whereas the mandible was not treated (“Untreated”). The animal was clinically examined at the indicated time points and the following clinical parameters were recorded: (A) gingival index; (B) bleeding on probing; (C) probing pocket depth; (D) clinical attachment level; (E) mobility index; and (F) plaque index. Figure S9. Effects of Cp40 on inflammatory clinical parameters of NHP chronic periodontitis (3X treatment): Raw data for monkey #9. Cp40 was injected – three times weekly for 6 weeks – into the interdental papillae and the distal gingiva of the second 7 molars of the maxilla (“Cp40”), whereas the mandible was not treated (“Untreated”). The animal was clinically examined at the indicated time points and the following clinical parameters were recorded: (A) gingival index; (B) bleeding on probing; (C) probing pocket depth; (D) clinical attachment level; (E) mobility index; and (F) plaque index. Figure S10. Effects of Cp40 on inflammatory clinical parameters of NHP chronic periodontitis (3X treatment): Raw data for monkey #10. Cp40 was injected – three times weekly for 6 weeks – into the interdental papillae and the distal gingiva of the second molars of the maxilla (“Cp40”), whereas the mandible was not treated (“Untreated”). The animal was clinically examined at the indicated time points and the following clinical parameters were recorded: (A) gingival index; (B) bleeding on probing; (C) probing pocket depth; (D) clinical attachment level; (E) mobility index; and (F) plaque index. Figure S11. Cp40 decreases inflammatory clinical parameters of naturally occurring chronic periodontitis in NHPs. Cp40 was injected once or three times weekly (1X and 3X treatments, respectively) into the interdental papillae and the distal gingiva of the second molars of the maxilla (“Cp40”), whereas the mandible was not treated (“Untreated”). The study involved Cp40 treatment for 6 weeks followed by 6 weeks during which the drug was discontinued. Each animal was clinically examined at the indicated time points and the following clinical parameters were recorded: (A) gingival index; (B) bleeding on probing; (C) probing pocket depth; (D) clinical attachment level; (E) mobility index; and (F) plaque index. The data were expressed relative to the baseline values (at time 0), set as 100 (raw data are shown for each animal in supplemental figures [S1 to S10 for 3X treatment and 8 S12 to S16 for 1X treatment]). Results are means ± SD (n = 5 monkeys for 1X treatment; n = 10 monkeys for 3X treatment). The data are from Figures 1 and 2 and are superimposed here for direct comparisons. Figure S12. Effects of Cp40 on inflammatory clinical parameters of NHP chronic periodontitis (1X treatment): Raw data for monkey #1. Cp40 was injected – once weekly for 6 weeks – into the interdental papillae and the distal gingiva of the second molars of the maxilla (“Cp40”), whereas the mandible was not treated (“Untreated”). The animal was clinically examined at the indicated time points and the following clinical parameters were recorded: (A) gingival index; (B) bleeding on probing; (C) probing pocket depth; (D) clinical attachment level; (E) mobility index; and (F) plaque index. Figure S13. Effects of Cp40 on inflammatory clinical parameters of NHP chronic periodontitis (1X treatment): Raw data for monkey #2. Cp40 was injected – once weekly for 6 weeks – into the interdental papillae and the distal gingiva of the second molars of the maxilla (“Cp40”), whereas the mandible was not treated (“Untreated”). The animal was clinically examined at the indicated time points and the following clinical parameters were recorded: (A) gingival index; (B) bleeding on probing; (C) probing pocket depth; (D) clinical attachment level; (E) mobility index; and (F) plaque index. Figure S14. Effects of Cp40 on inflammatory clinical parameters of NHP chronic periodontitis (1X treatment): Raw data for monkey #3. Cp40 was injected – once weekly for 6 weeks – into the interdental papillae and the distal gingiva of the second molars of the 9 maxilla (“Cp40”), whereas the mandible was not treated (“Untreated”). The animal was clinically examined at the indicated time points and the following clinical parameters were recorded: (A) gingival index; (B) bleeding on probing; (C) probing pocket depth; (D) clinical attachment level; (E) mobility index; and (F) plaque index. Figure S15. Effects of Cp40 on inflammatory clinical parameters of NHP chronic periodontitis (1X treatment): Raw data for monkey #4. Cp40 was injected – once weekly for 6 weeks – into the interdental papillae and the distal gingiva of the second molars of the maxilla (“Cp40”), whereas the mandible was not treated (“Untreated”). The animal was clinically examined at the indicated time points and the following clinical parameters were recorded: (A) gingival index; (B) bleeding on probing; (C) probing pocket depth; (D) clinical attachment level; (E) mobility index; and (F) plaque index. Figure S16. Effects of Cp40 on inflammatory clinical parameters of NHP chronic periodontitis (1X treatment): Raw data for monkey #5. Cp40 was injected – once weekly for 6 weeks – into the interdental papillae and the distal gingiva of the second molars of the maxilla (“Cp40”), whereas the mandible was not treated (“Untreated”). The animal was clinically examined at the indicated time points and the following clinical parameters were recorded: (A) gingival index; (B) bleeding on probing; (C) probing pocket depth; (D) clinical attachment level; (E) mobility index; and (F) plaque index. 10 Figure S17. Detection of osteoclasts in non-human primate periodontitis. The presence of osteoclasts on mesio-distal sections from bone biopsies was detected by TRAP staining. TRAP-positive cells are indicated by arrows. References Armitage, G. C. (2004) The complete periodontal examination. Periodontol 2000 34, 22-33. Bostanci, N., Ilgenli, T., Emingil, G., Afacan, B., Han, B., Toz, H., Atilla, G., Hughes, F. J. & Belibasakis, G. N. (2007) Gingival crevicular fluid levels of RANKL and OPG in periodontal diseases: implications of their relative ratio. J Clin Periodontol 34, 370-376. Laster, L., Laudenbach, K. W. & Stoller, N. H. (1975) An evaluation of clinical tooth mobility measurements. J Periodontol 46, 603-607. Loe, H. (1967) The Gingival index, the plaque index and the retention index systems. J Peridontol 38, Suppl:610-616. 11