Who Was Dueling in the Great Hall?
advertisement

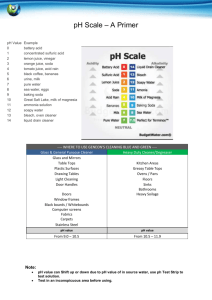
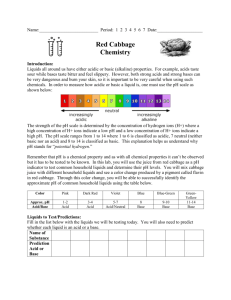
Teacher’s Instructions: Who Was Dueling In the Great Hall? Recommended grades: 3-6; estimated time: 1 hour Summary: Students will act as investigative chemists in this lab experiment to solve the mystery of who was dueling in Hogwart’s great hall. Using “wand core samples” students will experiment, observe, record, and identify trends to determine the unknown culprit. Common relatively inexpensive materials available from hardware, grocery, and drug stores are used so that this activity may be conducted on any budget. Two precipitation reactions, a pH indicator, and two visually stimulating reactions are used to engage students. After collecting data based on their spot plate experiments students are then expected to name the culprit and justify their accusations. Teaching Objectives: Communication: students will communicate effectively in the applications of science and technology. Scientific reasoning: students will learn to formulate and justify ideas and to make informed decisions. Inquiry and problem solving: Students will apply inquiry and problemsolving approaches in science and technology by observing and collecting data from their own experimental design Structure of Matter: Students will understand the structure of matter and the changes it undergoes Describe how physical properties of objects change during chemical reactions. Explain how matter changes in both chemical and physical ways Materials List: Corn Starch Available at most grocery stores in the baking isle. Iodine Available at most grocery stores as an antiseptic in the pharmacy. Acetic Acid White vinegar is available at most grocery stores with the condiments. Sodium Bicarbonate Baking soda from the baking isle of a grocery store. Magnesium Sulfate Commonly known as Epsom Salts, available at most drug stores. Sold as a topical anesthetic, laxative, or bath additive. Sodium Carbonate Washing soda is a common detergent-booster sold at most grocery stores in the laundry isle. Bleach A common cleaning agent, available at the grocery store in the laundry isle. Ben Bricker, Colby College, January 2012 Red Cabbage Juice Red cabbage from grocery store boiled for approximately 30 minutes and then strained to isolate the reddish-purple juice. Keep refrigerated. Used as a pH indicator. Calcium Hydroxide Commonly known as lime water, available at most health-food stores as a calcium supplement or pet stores as a reefaquarium supplement. Straws Grocery store. Spot plates Can be substituted with test tubes, cleaned out children’s paint sets, plastic ice cube trays, or plastic egg boxes. 5mL Pipettes Disposable pipettes are available cheaply in bulk from from any scientific equipment provider. Can also be replaced by straws. Safety Notice: All materials listed are safe to flush down the drain at the end of the lab Vinegar, bleach, and red cabbage all produce strong odors. Small, poorly ventilated classrooms should not conduct this experiment. Bleach and red cabbage juice will both permanently stain clothing. Preparing the Solutions: *All measurements are per 100mL of water Harry Potter: 1/8 tsp of Corn Starch and 1 Tbs Washing Soda Luna Lovegood: 20mL of Lime water and 1 Tbs Washing soda Hermione Granger: 10mL of Bleach and 1 Tbs Washing Soda Cedric Diggory: 1/8 tsp of Corn Starch and 1 Tbs of Baking Soda Ron Weasley: 20mL Lime Water and 1 Tbs Baking Soda Draco Malfoy: 10ml Bleach and 1 Tbs Baking Soda Epsom Salts: 1Tbs of Epsom salts in 100mL of water Allowing the solutions to sit for 10 minutes helps the baking soda mixture clear. Total prep time for the solutions ~40 minutes. Do not worry if some of the powders are left at the bottom of beaker after mixing. The entire powder does not need to completely dissolve into solution; 10-20 seconds of stirring with a disposable pipette should be sufficient. Experimental Setup: Give each group of 3-5 students 15mL test tubes with ~10mL of each suspect’s wand core sample along with an “unknown” test tube of the culprit of your choice. Four test tubes with 2-3mL of reactants should also be provided to each group. Preparing test tube racks with the necessary eight test tubes can be time consuming for the instructor (an additional 30-45 minutes due largely to test tube labeling), but cuts down the amount of time needed for the activity in the classroom while also limiting the potential hazard of students dropping, spilling, or confusing chemicals. Each group should also be provided with a spot plate to conduct their investigations and multiple disposable pipettes. Enough worksheets should also be given to the group so that each student may record their Ben Bricker, Colby College, January 2012 observations in the table provided. Allowing the students to have written directions to review as needed also helps the experiment to proceed more quickly. Beginning the Experiment: Begin by instructing students that today they are investigative chemists attempting to solve the mystery of who was dueling in Hogwart’s great hall. As chemists they will be working with chemicals and although everything they will be using is very diluted and relatively harmless, safety precautions must be observed. Everyone must wear safety goggles to protect their eyes and remember to handle their samples carefully. Demonstrating how to use the pipette to transfer a small amount of liquid from a test tube to the spot plate may be necessary if the class is unfamiliar with pipetting. Younger classes may wish to practice using their pipettes with water before moving on to the wand core and reactant samples. Reading aloud the prompt on the worksheet as a class and addressing any student questions before moving to individual efforts is recommended. Since all four student worksheets begin with the “unknown” core sample you may wish to conduct the first series of spot plate tests as a class so that students have a very clear idea of the procedure they are to follow for their next three samples. Allow the students to experiment and attempt to identify trends with between their samples. Leading questions such as “of the suspects, does anyone have a wand made of the same material?” or “do any of the reactions look similar to other tests you performed on someone else’s core sample?” may be necessary. Once a group of students believes they have identified the culprit (if they can with their wand core samples) ask them to explain their findings based on experimental evidence (for example the unknown reacted the same way as Harry’s wand core to iodine by turning blue, to Epsom Salts by creating a white cloud, and to red cabbage juice by turning blue. Nothing else reacted the same way to every substance). If students are struggling to match the samples it may be necessary to provide additional information. The prompt tells students that a thread was found at the scene of the duel, but does not tell them the color of the thread, nor the house colors of Hogwarts. Depending on perpetrator match the color to their house: House Student(s) Colors Gryffindor Harrry, Hermione, Ron Crimson & Gold Slytherin Draco Green & Silver Ravenclaw Luna Blue & Bronze Hufflepuff Cedric Yellow & Black Once the majority of students have finished their spot plate tests have students compile their data. Drawing a grid on a marker board and recording their observations can help the class to identify the culprit. The entire eight by six grid does not need to completed; instead jump to the most important reactions with the most vivid indicators. Harry’s sample turns black and develops a precipitate when iodine is added, and forms a white precipitate when Epsom salts are added. Hermione’s sample turns blue when red cabbage juice is added and forms a white precipitate when Epsom salts are added. By filling in only the most important reactions the chart will be less cluttered, and the pattern will be easier for students to identify. Ben Bricker, Colby College, January 2012 Reactions: Vinegar and baking soda react to produce water, carbon dioxide, a salt, and heat NaHCO3(aq) + HC2H3O2(aq) ===> NaC2H3O2(aq) + CO2(g) + H2O(l) Epsom salts and washing soda dissolved in water produce a precipitate, magnesium carbonate. MgSO4(aq) + Na2CO3(aq) ===> MgCO3(s) Corn starch and iodine react to produce a dark blue complex. Beta amylose + I2(aq) ==> I5-1 (a dark blue complex) Red cabbage juice is a natural pH indicator due to the presence of anthocyanin, a pigment that is pH sensitive. Red cabbage juice is blue in strongly basic pH, greenish in neutral pH, and deep red in strongly acidic pH. Lime water when exposed to the carbon dioxide from a person’s breath (in this case through a straw) will slowly form a white precipitate that will make the solution cloudy. Having students run in place, or conducting the experiment after gym class/recess will also make the reaction more effective. Dry ice may also be used, but only in small amounts. Too much dry ice will form a precipitate, dissolve the precipitate, and leave the solution acidic. CO2(g) + Ca(OH)2(aq) ===> CaCO3(s) Reference: US Department of Energy. Newton Archives. April 2011. January 2012 <http://www.newton.dep.anl.gov/> Millard, Jullie. "CH151: k-8 Chem Outreach." January 2012. Colby College Department of Chemistry Course Web Pages. January 2012 <http://www.colby.edu/chemistry/CH151_current/CH151_current.html>. Ben Bricker, Colby College, January 2012 Name: Date: Who Was Dueling in the Great Hall? Student Handout Introduction: Someone was dueling in the Great Hall of Hogwarts last night, and Professor McGonagall has turned to your well-respected muggle chemistry department to determine who it was. Professor McGonagall found an unknown sample of what she believes to be part of a wand core at the scene of the duel. She also has collected wand core samples from all students out of bed after curfew from the night in question. A thread, likely from a students’ house scarf was also found at the scene. Since muggles rarely are able to work with substances such as a hair from a unicorn’s tail or a dragon’s heartstring you will first need to conduct a series of investigative tests on the wand cores to determine what these magical substances react with. You will be given four test tubes labeled by student, spot plates to conduct your experiments, and several reactants to explore the chemical properties of your samples. Observing and recording the results of your experiments on each student’s wand core are key. Record such observations as color change, bubbling, or if your solution creates a solid. Due to the large number of suspects each group will be given four students to investigate. After experimenting and recording your observations you will determine, as a class, who was dueling in the great hall. Procedure: 1. Using your Pipette add 10-15 drops of a wand core sample to four spot-plate wells. 2. Add a few drops of Iodine to your first spot plate well and record your observations in the table provided. 3. Add a few drops of Red Cabbage Juice to the next spot plate well. Again, record your observations. 4. Add Vinegar to your third spot plate well. Record any observations in the data table. 5. Add Epsom Salts to your fourth and final spot plate well. Record any observations in the data table. 6. Place your straw in the test tube and gently blow bubbles through the solution for 20 seconds. Record any observations in your data table. 7. Repeat steps 1-6 for each student suspected of dueling. Ben Bricker, Colby College, January 2012 Raise your hand and sit quietly to show that your group has finished their experiments and is ready to share with the class. 1. Reactant: Adding Iodine Adding Red Cabbage Juice Student: Unknown Duelist Unknown wand core/ Unknown Wood Harry Potter Phoenix feather/ Vinewood Hermione Granger Dragon Heartstring/ Vinewood Ron Weasley Unicorn Hair/ Hawthorn wood Ben Bricker, Colby College, January 2012 Adding Vinegar Adding Epsom Salts Adding Carbon Dioxide (through a straw) Raise your hand and sit quietly to show that your group has finished their experiments and is ready to share with the class. 2. Reactant: Adding Iodine Adding Red Cabbage Juice Student: Unknown Duelist Unknown wand core/ Unknown Wood Draco Malfoy Dragon Heartstring/ Hawthorn wood Luna Lovegood Unicorn hair/ Vinewood Cedric Diggory Phoenix feather/ Hawthorn wood Ben Bricker, Colby College, January 2012 Adding Vinegar Adding Epsom Salts Adding Carbon Dioxide (through a straw) Raise your hand and sit quietly to show that your group has finished their experiments and is ready to share with the class. 3. Reactant: Adding Iodine Adding Red Cabbage Juice Student: Unknown Duelist Unknown wand core/ Unknown Wood Ron Weasley Unicorn Hair/ Hawthorn wood Draco Malfoy Dragon Heartstring/ Hawthorn wood Luna Lovegood Unicorn hair/ Vinewood Ben Bricker, Colby College, January 2012 Adding Vinegar Adding Epsom Salts Adding Carbon Dioxide (through a straw) Raise your hand and sit quietly to show that your group has finished their experiments and is ready to share with the class. 4. Reactant: Adding Iodine Adding Red Cabbage Juice Student: Unknown Duelist Unknown wand core/ Unknown Wood Harry Potter Phoenix feather/ Vinewood Hermione Granger Dragon Heartstring/ Vinewood Cedric Diggory Phoenix feather/ Hawthorn wood Ben Bricker, Colby College, January 2012 Adding Vinegar Adding Epsom Salts Adding Carbon Dioxide (through a straw) Ben Bricker, Colby College, January 2012