HL Chemistry Lab Report Format
advertisement

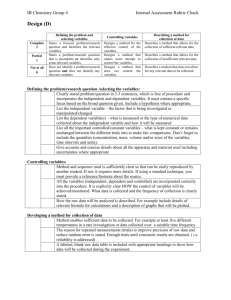
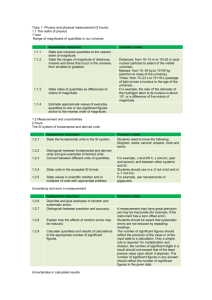
S. SMITH IB HL Chemistry Lab Report Format When you are submitting a lab for DATA COLLECTION & PROCESSING as well as CONCLUSION AND EVALUATION criteria only, focus on the following areas. Keep in mind the criteria outlined in both the official and expanded rubrics. NAME: DATE: Your title should reveal both your independent and dependent variable . be descriptive… give the reader an idea of what is to come. TITLE: GROUP/PARTNER: RESEARCH QUESTION: HYPOTHESIS: VARIABLES: PURPOSE / PROCEDURE: Clearly identify the purpose of doing the lab. Then summarize the method in paragraph form and cite the source. In this situation there is no need to list materials or rewrite the actual procedure. You should identify the chemical reactions taking place – it helps provide the context for the lab. DATA COLLECTION: The data you record as you are doing your lab is considered ‘raw, raw’ data. Although having an organized table in advance to record raw raw data in is a good idea, it is not required. Your instructor may ask that you get raw raw data initialed. Collecting and recording of raw data must include qualitative as well as quantitative data, including units and uncertainties where necessary. Once you have completed initial data collection, organize the data so that it is clearly presented, allowing for easy interpretation. Use of one or more tables for raw data is expected. Include all relevant data, even if mentioned in the procedure. Give an identifying number and title to each table. Organize observations into rows and columns for greatest efficiency and clarity. If there are any difficulties (‘spills’, ‘time constraints’) encountered in carrying out the stated method note them in this section. DATA PROCESSING & PRESENTATION: CALCULATIONS OF RESULTS: Select an appropriate method to process the data. This may involve calculations, graphing or other forms of analysis. Do one sample calculation; identical calculations do not need to be repeated. Include any equations you use, show all steps clearly and explain the methods used. Often it is preferable to set up an equation for an entire calculation, rather than carrying it out in separate steps. This avoids rounding errors and reduces the likelihood of errors resulting from stepwise calculations. Use significant figures appropriately. The final result should be consistent with the number of significant figures in the experimental measurements and any subsequent calculations based on them. For repeated trials, calculate a final result for each trial; do not use an average of raw data. Calculate an average result, based on final results of repeated trials. CALCULATIONS OF ERRORS AND UNCERTAINTIES: Use final results of repeated trials for calculation of experimental uncertainty. Uncertainties should be estimated for single measurements and carried throughout the calculations. They can be stated as absolute or relative values. Errors should be calculated where possible (percent error, percent yield), using the final average value. These two values – the uncertainty and the experimental error need to be compared in the evaluation. PRESENTATION OF RESULTS: This must be easy to follow and understand, comprehensive, and appropriate to the nature of the results. Errors and uncertainties should be noted, when relevant. Graphs will often be utilized, especially for several values of continuous variables. When constructing graphs, be sure to show a title and label the axes, use an appropriate size (not too small), use an appropriate scale, indicate points clearly, and show the relationship by fitting points to line(s) or a smooth curve. If graphs are not appropriate, summarize results in a table. CONCLUSION: Begin with a few summary statements that highlight the results of your investigation. Then interpret the results and state a valid conclusion. Your conclusion should be clearly related to the purpose of the experiment. It should address your hypothesis, if you stated one. Explain how your conclusion follows from the results, directing the reader to specific evidence to support the conclusions. Provide explanations of results obtained. Compare results with literature or accepted values (please cite these resources), and calculate percent error when appropriate. EVALUATION: SOURCES OF ERROR: Use this section to analyze all relevant sources of error. Do not be vague and do NOT use the phrase ‘human error’. List pertinent errors and specifically indicate how each one might have impacted the results. In your analysis, evaluate the procedures and results. For procedures, consider the following questions: Are there flaws in the procedures which could have affected the results? Were important variables not controlled? Were measurements and observations reliable? Is precision unknown because of lack of replication? [These would be considered systematic errors and address limitations of the investigation.] For results, consider these questions: What is the uncertainty of the results and how does it impact any conclusions made? [Uncertainty is considered random error.] How does the uncertainty compare to the actual experimental error (% error, % yield). If the experimental error is greater than the random error, account for any differences. If there were experimenter errors, you may discuss them – but only if they first appear in your raw data. SUGGESTIONS FOR IMPROVEMENT: Suggestions must be made on how to improve the investigation according to weaknesses identified in “sources of error”. Suggestions should be realistic, not involving unavailable equipment or materials. They should be specific and not vague (e.g. “more careful work”). Proposed changes may do any or all of the following: eliminate or reduce errors, improve control of variables, reduce approximations, and/or provide other procedures for better measurements. State if modifications are unnecessary or impossible (i.e. standard or prescribed procedures were used).