Supporting Information - Springer Static Content Server
advertisement

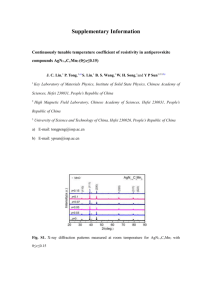
Supporting Information A turn-on fluorescent chemosensor for Zn2+ based on quinoline in aqueous media Yong Sung Kim,a Jae Jun Lee,a Sun Young Lee,a Pan-Gi Kim,b Cheal Kima* a Department of Fine Chemistry and Department of Interdisciplinary Bio IT Materials, Seoul National University of Science and Technology, Seoul 139-743, Republic of Korea. Fax: +82-2-973-9149; Tel: +82-2-970-6693; E-mail: chealkim@seoultech.ac.kr b School of Ecology & Environmental Systems, Kyungpook National University, Sangju 37224, Korea (a) 1 Fig. S1. Fluorescence spectra of 1 (20 µM) with Zn2+ in various solvents. 2 Fig. S2. Job plot of 1 and Zn2+ in a mixture of MeCN/bis-tris buffer solution (3:7, v/v). The total concentrations of 1 and Zn2+ were 50 μM. 3 Fig. S3. Benesi-Hildebrand equation of 1 (at 523 nm), assuming 1:1 stoichiometry for association between Zn2+ and 1. 4 Fig. S4. Detection limit of 1 (20 µM) for Zn2+ through change of fluorescence intensity. 5 (a) 0.8 Absorbance experiment theoretical 0.6 0.6 0.4 0.4 0.2 0.2 0.0 280 350 Oscillator strength 0.8 0.0 490 420 Wavelength (nm) (b) Excited State 1 Wavelength Percent (%) Oscillator strength H→L 332.82 nm 58 % 0.0931 H-1 → L 40 % Excited State 2 Wavelength Percent (%) Oscillator strength H-1 → L 328.21 nm 56 % 0.0664 H→L 41 % Fig. S5. The theoretical excitation energies and the experimental UV-vis spectrum of 1. (b) The major electronic transition energies and molecular orbital contributions for 1 (H = HOMO and L = LUMO). 6 Fig. S6. Molecular orbital diagrams of 1 and 1-Zn2+, and their excitation energies by TD-DFT methods. 7 0.8 Absorbance experiment theoretical 0.6 0.6 0.4 0.4 0.2 0.2 0.0 280 350 Oscillator strength 0.8 0.0 490 420 Wavelength (nm) (a) (b) Excited State 1 Wavelength Percent (%) Oscillator strength H → L+1 430.35 nm 80 % 0.0284 H→L 19 % Excited State 3 Wavelength Percent (%) Oscillator strength H-1 → L 330.48 nm 96 % 0.1317 Fig. S7. (a) The theoretical excitation energies and the experimental UV-vis spectrum of 1Zn2+. (b) The major electronic transition energies and molecular orbital contributions for 1Zn2+ (H = HOMO and L = LUMO). 8















![De novo design of a transmembrane Zn[superscript 2+]- transporting four-helix bundle](http://s2.studylib.net/store/data/011847263_1-ba83c4e46f9805e47e217c678835e777-300x300.png)

