Kinetic Calculation of Pressure
advertisement
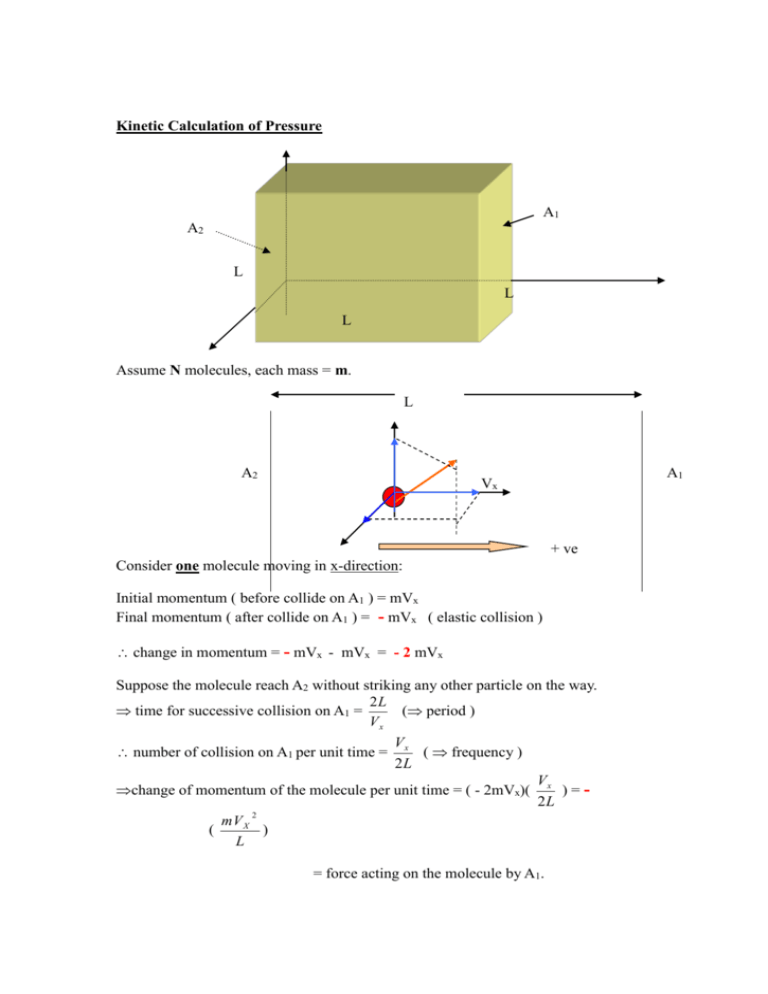
Kinetic Calculation of Pressure A1 A2 L L L Assume N molecules, each mass = m. L A2 A1 Vx + ve Consider one molecule moving in x-direction: Initial momentum ( before collide on A1 ) = mVx Final momentum ( after collide on A1 ) = - mVx ( elastic collision ) change in momentum = - mVx - mVx = - 2 mVx Suppose the molecule reach A2 without striking any other particle on the way. 2L time for successive collision on A1 = ( period ) Vx V number of collision on A1 per unit time = x ( frequency ) 2L V change of momentum of the molecule per unit time = ( - 2mVx)( x ) = 2L 2 mV X ( ) L = force acting on the molecule by A1. By Newton's 3rd Law: 2 mV X force on A1 by the molecule = + ( ) L 2 pressure on A1 due to this molecule = p = F =( A m( For N- molecules, total pressure on A1: P = p1 + p2 + p3 + p4 + = mVX 1 2 L3 + mVX 2 L3 VX ) 2 L ) = mV X L2 L3 + pN 2 + mVX 3 2 + L3 + mVX N 2 L3 Where Vxi = x-component of velocity of ith molecule. P= m ( Vx12 + Vx22+ Vx32 + L3 + VxN2 ) N = number of molecule per unit volume = particle density L3 N L3 = n 2 2 2 2 m(V X 1 V X 2 V X 3 V XN ) P= N n 2 2 2 2 (V X 1 V X 2 V X 3 V XN ) =(nm) = V X2 ( = density of gas N If n = average value of (VX)2 = V X2 inside container ) V2 = V 2X VY2 VZ2 V 2 V X2 VY2 VZ2 V2 For random motion : V V V 3 2 X Hence, P = 1 V2 3 or 2 Y P= 2 Z 1 Nm 2 V 3 or Pv = 1 NmV 2 3 Further: nM v & Pv = nRT = (1) M = molar mass of gas n = no. of moles of gas (2) ( 2) P nRT RT (1) nM M V2 3P 3RT M root-mean-square velocity ( V RMS ) e.g. Find the Vrms of oxygen gas at 27 0 C. ( given that the molecular mass of oxygen gas = 32 ) Ans: ~ 480 ms -1 Kinetic interpretation of temperature Pv = 1 1 NmV 2 nM V 2 3 3 Pv = 2 1 nM V 2 3 2 total K.E. of the molecules. ( called the thermal energy / internal energy ) But, Pv = nRT 2 1 nM V 2 = nRT 3 2 3 1 M V 2 = RT 2 2 ( = K.E. per mole ) Divide both side by N ( avogadro’s number ) 1 M 2 3 R V ( )T 2 N 2 N Boltzman constant, k = 1.38x10-23 J / molecule.K 1 3 mV 2 kT 2 2 Hence, average K.E. per molecule T ( K ) different ideal gases have the same average K.E. per molecule at equal temperature.









