Lab: Investigating LD50: How much is too much? Background
advertisement

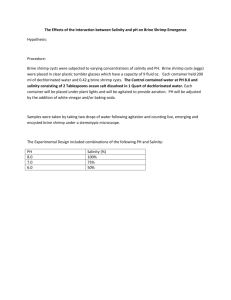
Lab: Investigating LD50: How much is too much? Background Information In 1972, the U.S. federal government began maintaining water quality with the passage of the Federal Water Pollution Control Act. Better known as the “Clean Water Act” (CWA), it provides funding for local and state programs for the protection of surface waters. Its objective is to maintain the “chemical, physical, and biological integrity of the nation’s waters.” There are two major categories of pollution: point source and nonpoint source. Point sources are specific places from which pollutants are discharged, e.g., a factory or a malfunctioning sewage treatment plant. Factories such as pulp and paper mills, refineries, and automobile manufacturers typically release pollutants in their discharge water (also known as effluent). It was once common that these effluents were released directly into a nearby body of water. At present, many factories treat their industrial wastewater on site before it is released, while others send their effluent to a sewage plant to be treated. The role of the Clean Water Act has been to regulate and maintain strict control of the discharges. Each point source is required to have a permit as well as the latest technology for treatment of its effluent. Nonpoint source pollution is difficult to control because it comes from a wide range of sources. It is caused by runoff from rainfall or snowmelt moving over the ground. As the water moves, it picks up natural and human-produced (anthropogenic) debris and pollutants and deposits them directly into lakes, rivers, and wetlands or into the groundwater as it percolates through soil. These pollutants may include Sediment from construction sites Excessive fertilizers and pesticides Oil and grease from urban runoff Bacteria from livestock or pet wastes (or human) Understanding the areas and situations likely to contribute to nonpoint source pollution helps scientists and engineers develop ways to prevent it before it occurs. For example, sediment fences are used at construction sites to catch debris and soil particles and to slow runoff. Retention ponds are also created to capture storm water and contaminants and to control the rate of runoff. Runoff is reduced through the use of porous paving materials on roadways and parking lots, where water is allowed to seep into the ground. Such techniques may be mandated through a variety of state and local regulations and codes, some of which were developed to meet the requirements of federal legislation and others of which may go beyond those requirements. An LD50 experiment, which determines the lethal dose that kills 50% of a test population of organisms, is used to test the toxicity of certain chemicals. This test provides useful data to establish regulations on how much of a certain chemical can be discarded into the environment before it becomes lethal. Many times, experiments to determine the LD50 of chemicals and toxins are carried out on small rodents and other mammals, because their physiology is so closely related to humans. Ultimately, that is what we are concerned with as well, as anthropocentric as that sounds! The EPA (Environmental Protection Agency) requires an LD50 on pesticide labels, and provisions of the Clean Water Act set limits on discharges of a large number of chemicals. In your lab groups, you will perform an LD50 test using brine shrimp or amphipods. You will have choices as to what toxin, or chemical pollutant you use. Lab Objective: The goal of the lab is to determine the LD50 of various compounds (of your choice) you will introduce to a colony of brine shrimp or amphipods. This is a common toxicology test where a known concentration is applied to a sample population. The concentration level that kills 50% of the population is referred to as the LD50. Background on Brine Shrimp Artemia (genus of brine shrimp) is a typical primitive arthropod with a segmented body. The body usually consists of 19 segments, the first 11 of which have pairs of appendages, the next two which are often fused together carry the reproductive organs, and the last segments lead to the tail. The total length is usually about 8–10 millimetres for the adult male and 10–12 mm for the female. The body of Artemia is divided into head, thorax, and abdomen. The entire body is covered with a thin, flexible exoskeleton composed of chitin to which muscles are attached internally and shed periodically. For brine shrimp, many functions, including swimming, digestion and reproduction are not controlled through the brain; instead, local nervous system ganglia may control some regulation or synchronization of these functions. Autotomy, the voluntary shedding or dropping of parts of the body for defense, is also controlled locally along the nervous system. Day One: Observe and Collect data This is an inquiry lab. You will be given some brine shrimp to observe. Watch them and document observations of their behavior in a “T” Chart, “I notice…I wonder…”. Use a magnifying glass to help you see them. What types of behavior do you observe? Do they respond to tapping vibrations? Vocalizations? Time how long they swim. Can you see their heart beating with the magnifying glass? Do they appear to be social, grouping together, or solitary? Do the larger ones move more often than the smaller ones? Which ones are faster? Use your imagination. Get to know your brine shrimp, because you will be watching them closely once the experiment starts, and need to understand their responses. In your lab groups today, you will determine the toxin to be introduced into your colony. If you are planning on providing your own chemical/toxin, please me know what it is you are planning to bring! The following are toxins that I can provide for you: Copper sulfate (common component of herbicides and algaecides) Chlorox NaCl Common household cleanser Household wall paint Motor oil Ethyl Alcohol Borax Gasoline Diazinon Acetone Brainstorm in your group about some common household chemicals that you might test. Think about any chemical used around the house, hot just cleaning agents. Choose one chemical to investigate. If you can think of any other toxin that might prove dangerous when released into the environment, that I might have, or could get my hands on, simply ask. Day Two: Plan your lab. By the end of class today, you should have: Written the title of the lab Written the question your group is investigating Written your first 2-3 procedures Written your materials list Written up your safety precautions Designed any data tables to collect quantitative and qualitative data Written down your experimental design Prepared your solutions. You will need the following: The substance you are planning on polluting the brine shrimp environment with A 20% solution A 2% solution A .2% solution .02% solution Please note…this is strictly an “observation” type lab. NO HYPOTHESIS is necessary! To create a 20% solution of a solid/liquid Measure 20 gm of the solute/or 20 mL if it is a liquid Add it to a 100 mL graduated cylinder Add enough ocean water (brine shrimp medium) to meet the 100 mL line on the graduated cylinder. (That should be roughly 80 mL if you are diluting a liquid) To create a 2% solution: (Using your 20% solution stock) Pour 60 mL of 20% solution into a 1,000 mL graduated cylinder Add 540 mL of ocean water (brine shrimp medium) to create 600 mL of 2% solution To create a .2% solution: (Using your 2% solution stock) Pour 60 mL of 2% solution into a 1,000 mL graduated cylinder Add 540 mL of ocean water (brine shrimp medium) to create 600 mL of .2% solution Now…let’s see if you can figure out how to make a .02% solution! Day 3: Carry out your plan, and make any necessary changes. Collect data. Get ready to repeat and collect more data tomorrow, if necessary Analysis Questions: 1. What was the LD50 of your toxin, if you were able to determine that…and if you weren’t able to, describe how you might continue your experiment, in order to better determine the LD50. 2. Poll the other 5 lab groups in your class, to determine the LD50s of their toxins. Create a data table to display this information. Which toxin was able to kill in the least concentrated dose? 3. Think of ONE INVESTIGABLE question this lab made you consider. Write it here. Remember, avoid “why” questions! Don’t forget error analysis, and reflection!