tpj12851-sup-0010-MethodsS1
advertisement

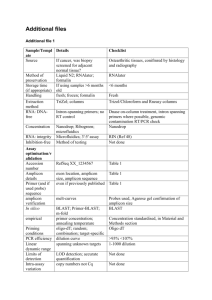
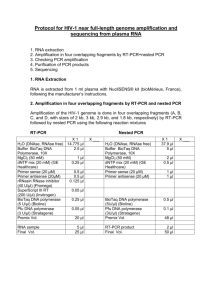
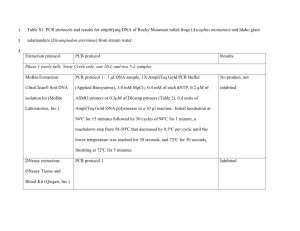
Methods S1 PCR conditions for 454 amplicon and Sanger sequencing In 454 amplicon sequencing pcr amplification was carried out using the forward primer aGli900F1 (5’-GTTAGAGTTCCAGTGCCACAA-3’; for VRVPVPQ motif, Figure 1) and the reverse primers MJ_R6 (5’-ACATCCMTGCATGGAATCA-3’; for IPC[M/R]D motif) and MJ_R3 (5’-TGGCACYGCGACTGCTC-3’; for EQS[Q/R]C motif) with following conditions: 94ºC for 1 min followed by 30 cycles at 94ºC for 15 s, 62ºC for 45 s and 72ºC for 1 min with final extension at 72ºC for 2 min. For PCR amplification was used, 5 ng of DNA in a 25 uL volume reaction with the following final concentrations: 1x FastStart buffer, 200 nM forward and reverse primers, 200 uM dNTP mix, 1.25 units of FastStart High Fidelity polymerase (Roche Diagnostics, Mannheim, Germany). The gene-specific primers for Sanger sequencing were as follows, forward primer VH_agliF1 (5’-ATGAAGACCTTTCTCATCC-3’; for MKTFLI motif, Figure 1) and reverse primers VH_agliR3 (5’-GTTRGTACCGAAGATGCC-3’; for GIFGTN motif). These primers amplified from signal peptide in the 5’ to end of the coding region in the 3’ as described (van Herpen et al., 2006). The R3 primer was designed based on the sequences of the α-gliadins present in the NCBI database. The complete α-gliadin gene were amplified by PCR as follow: 94ºC for 4 min followed by 35 cycles at 94ºC for 15 s, 52ºC for 1 min and 72ºC for 1 min with final extension at 72ºC for 7 min. For PCR amplification was used, 1 ug of DNA in a 25 uL volume reaction consisting of 300 nM forward and reverse primers, 320 uM dNTP mix, a mixture of 0.013 units Pfu DNA polymerase (Biotools, B&M Labs, Madrid, Spain) and 0.650 units Taq DNA polymerase (Biotools). PCR products were checked by 1% agarose gel. van Herpen, T.W., Goryunova, S.V., van der Schoot, J., Mitreva, M., Salentijn, E., Vorst, O., Schenk, M.F., van Veelen, P.A., Koning, F., van Soest, L.J., Vosman, B., Bosch, D., Hamer, R.J., Gilissen, L.J. and Smulders, M.J. (2006) Alpha-gliadin genes from the A, B, and D genomes of wheat contain different sets of celiac disease epitopes. BMC genomics, 7, 1.