Honors Chemistry Midterm Study Guide Students will be required to
advertisement

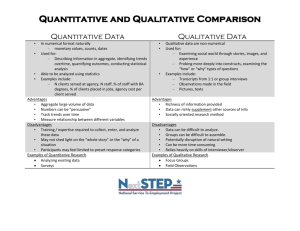
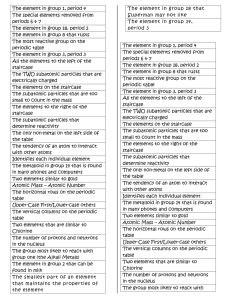
Honors Chemistry Midterm Study Guide Students will be required to answer questions based on the following topics listed. Students will not need to answer any questions on topics not listed on this sheet. Students may be required to answer a variety of types of questions on all/any topics including, but not limited to: multiple choice, short answer, fill-in the blank, quantitative reasoning, qualitative reasoning, etc. The test is designed to take 90 minutes during the 120 minute testing period. Students will each receive a copy of the periodic table and an electromagnetic spectrum. Topics: 1. Significant Figures identification, calculation, reading measuring tools 2. Density qualitative & quantitative (formula given) 3. Metric Prefixes and Dimensional Analysis quantitative (must have pico to terra memorized) 4. Classification of Matter Elements, compounds, mixtures i. illustrations Homogeneous vs. Heterogeneous Solution vs. Colloid vs. Suspension 5. Properties Physical vs. Chemical Intensive vs. Extensive (Intrinsic vs. Extrinsic) 6. Changes Physical vs. Chemical Signs of Chemical Changes Basic Setup of Chemical Equations/Reactions 7. Law of Conservation 8. Atomic Theory Thomson & Rutherford experimentation, models, and discovery Modern Atomic Model Quantum #’s Atom Energy Levels, Sublevels, and electron occupancy 9. Electron Configurations Complete Configurations Abbreviated Configurations 10. Waves Qualitative and Quantitative understanding of Energy, Wavelength, Frequency (all constants and equations given) 11. Light Theory of light emission Atomic Spectra used to analyze 12. Subatomic Particles Counting/Identifying Subatomic Particles Isotopes Average Atomic Mass (equation not given on test) 13. Periodic Table Periodic Trends (Electronegativity, Ionization Energy, Atomic Radius) # valence electrons ion formation based on # valence electrons Representative elements 14. Counting Atoms in Compounds 15. Moles & Mole Calculations All constants and equalities must be memorized and will not be given on test. 16. Creating Appropriate Data Tables 17. Identifying controls, independent and dependent variables in experiments.