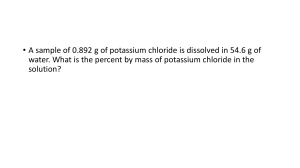AP Chemistry
advertisement

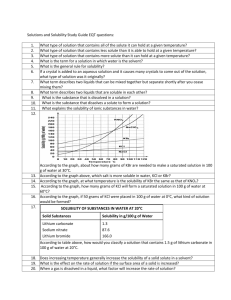
Honors Chemistry Mrs. Klingaman Ch. 13: Properties of Solutions Objectives: The student will be able to: Understand solutions concentration (molarity/molality) Understand the solution process at the molecular level, including the role of intermolecular forces. Understand the difference between unsaturated, saturated and supersaturated solutions. Understand the factors affecting solubility. Be able to interpret a curve relating solubility as a function of temperature. Be able to express solution concentration according to mass percentage, mole fraction, molarity and molality. Understand colligative properties of solute particles in solution. 4.5 – Concentrations of Solutions (pg. 144) 1. Define concentration as it relates to solutions. What is a solute? Solvent? 2. Define molarity and units used to describe it. 3. How does solutions concentration apply to electrolytes? 4. Identify the relationship between molarity, moles and volume. 5. What formula is used to help describe dilution of a solution? 13.1 - The Solution Process (pg. 530) 1. Define the terms solution, solvation, and hydration. 2. Review Figure 13.4 and the supporting text. Why is the associated enthalpy change during solvation exothermic in some cases and endothermic in others? 3. Write an equation to describe the solution process. How does this differ from a chemical equation? (Hint: look at pg. 534 “A Closer Look – Hydrates” and sample exercise 13.1) 13.2 - Saturated Solutions and Solubility (pg. 535) 4. Explain the dynamic equilibrium created when solute dissolves/recrystallizes. 5. Distinguish between unsaturated, saturated and supersaturated solutions. 13.3 - Factors Affecting Solubility (pg. 537) 6. Based on your understanding of intermolecular attractions, what is the role of these forces on solute-solve interactions? 7. Define and differentiate between the terms miscible and immiscible. 8. Explain the phrase, “like dissolves like”. 9. What is the role of pressure on the solubility of a solid/liquid solutions and a gas/liquid solution? Include Henry’s law in your answer. 10. What is the role of temperature on the solubility of gasses and solids in liquids? 13.4 - Expressing Concentration (pg. 543) 11. What is meant by parts per million (ppm) and when is it most applicable? 12. Identify the equation to describe solution concentration based on Molarity. 13. Identify the equation to describe solution concentration based on Molality. 14. Review the flow chart described in Figure 13.19 that allows for conversions between solution molarity and molality. 13.5 - Colligative Properties (pg. 548) 15. What is meant by the term colligative properties? 16. Explain how vapor pressure is impacted by solute concentration. Include Raoult’s law in your explanation. 17. Using the phase diagram described in figure 13.22, explain why the freezing point is depressed and boiling point is elevated when solute is added to a solvent. 18. Identify ΔTb and ΔTf and the equations used to define them. 19. What is osmosis? (understand concepts behind this process)