therapy allergic
advertisement
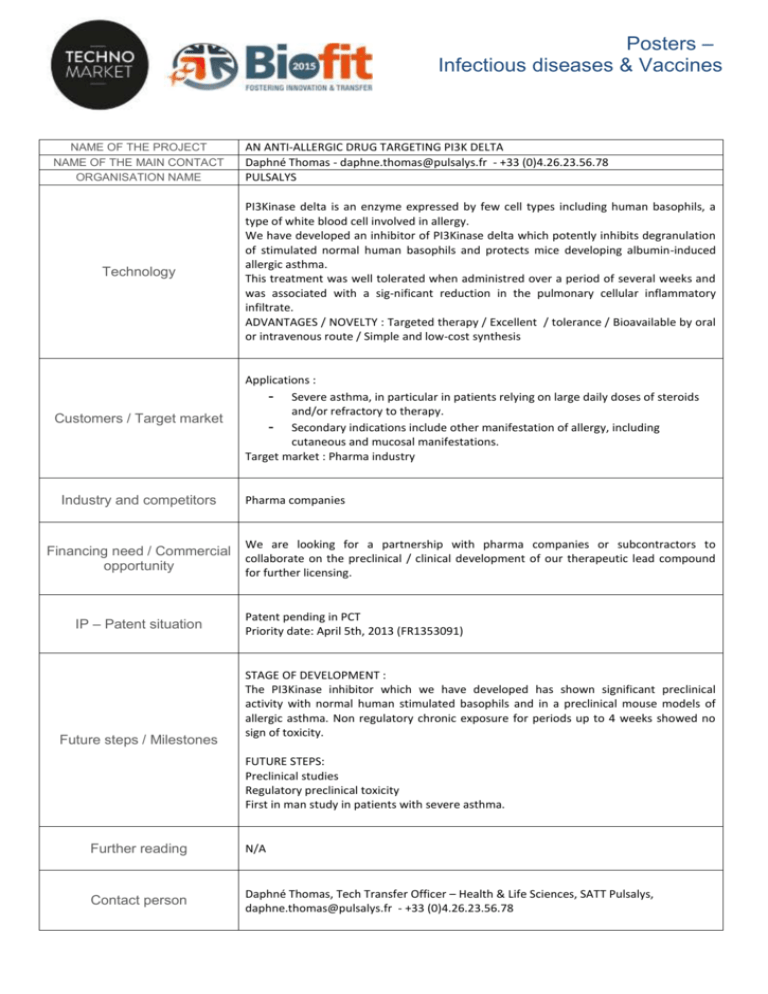
Posters – Infectious diseases & Vaccines NAME OF THE PROJECT NAME OF THE MAIN CONTACT ORGANISATION NAME Technology Customers / Target market Industry and competitors Financing need / Commercial opportunity IP – Patent situation Future steps / Milestones AN ANTI-ALLERGIC DRUG TARGETING PI3K DELTA Daphné Thomas - daphne.thomas@pulsalys.fr - +33 (0)4.26.23.56.78 PULSALYS PI3Kinase delta is an enzyme expressed by few cell types including human basophils, a type of white blood cell involved in allergy. We have developed an inhibitor of PI3Kinase delta which potently inhibits degranulation of stimulated normal human basophils and protects mice developing albumin-induced allergic asthma. This treatment was well tolerated when administred over a period of several weeks and was associated with a sig-nificant reduction in the pulmonary cellular inflammatory infiltrate. ADVANTAGES / NOVELTY : Targeted therapy / Excellent / tolerance / Bioavailable by oral or intravenous route / Simple and low-cost synthesis Applications : - Severe asthma, in particular in patients relying on large daily doses of steroids and/or refractory to therapy. - Secondary indications include other manifestation of allergy, including cutaneous and mucosal manifestations. Target market : Pharma industry Pharma companies We are looking for a partnership with pharma companies or subcontractors to collaborate on the preclinical / clinical development of our therapeutic lead compound for further licensing. Patent pending in PCT Priority date: April 5th, 2013 (FR1353091) STAGE OF DEVELOPMENT : The PI3Kinase inhibitor which we have developed has shown significant preclinical activity with normal human stimulated basophils and in a preclinical mouse models of allergic asthma. Non regulatory chronic exposure for periods up to 4 weeks showed no sign of toxicity. FUTURE STEPS: Preclinical studies Regulatory preclinical toxicity First in man study in patients with severe asthma. Further reading N/A Contact person Daphné Thomas, Tech Transfer Officer – Health & Life Sciences, SATT Pulsalys, daphne.thomas@pulsalys.fr - +33 (0)4.26.23.56.78