Millikan Oil Drop Apparatus Simulation
advertisement
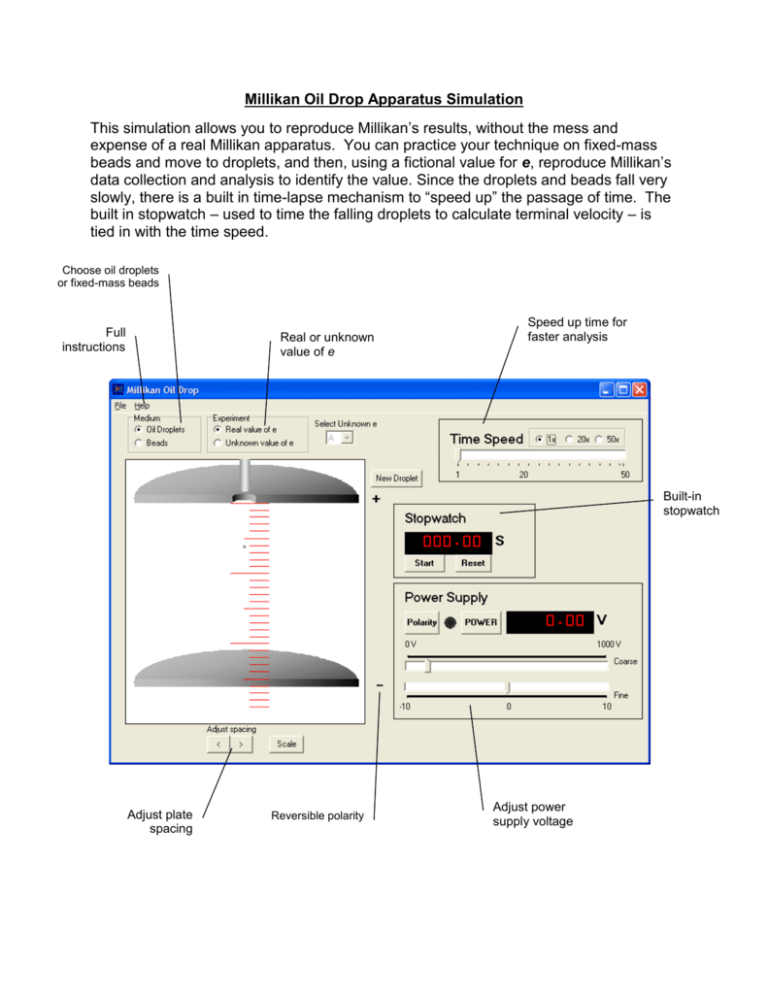
Millikan Oil Drop Apparatus Simulation This simulation allows you to reproduce Millikan’s results, without the mess and expense of a real Millikan apparatus. You can practice your technique on fixed-mass beads and move to droplets, and then, using a fictional value for e, reproduce Millikan’s data collection and analysis to identify the value. Since the droplets and beads fall very slowly, there is a built in time-lapse mechanism to “speed up” the passage of time. The built in stopwatch – used to time the falling droplets to calculate terminal velocity – is tied in with the time speed. Choose oil droplets or fixed-mass beads Full instructions Real or unknown value of e Speed up time for faster analysis Built-in stopwatch Adjust plate spacing Reversible polarity Adjust power supply voltage Using the Millikan Oil Drop Simulation The experiment: The apparatus for this experiment was developed by Millikan and Fletcher in 1909 to determine the charge of an individual electron. The experiment is both simple and elegant. If a droplet of oil can be suspended between charged plates, then the upward electric force on the droplet exactly matches the downward force of gravity on the droplet. If the mass of the droplet can be found, then the charge on the droplet can be found. This experiment is often performed with microscopic spheres of known mass, but originally the mass of oil droplets was determined by timing the fall of the droplet, and using the density and terminal velocity to find the mass. Either method may used in this simulation. Why use a simulation? Performing this experiment in the classroom or lab using real equipment is daunting for many instructors. It is time consuming to perform the experiment and the high voltage can be dangerous. In addition, the equipment must be cleaned and maintained. Using the simulation, set up and clean up times are non-existent, and there is no danger of electrocution. In this simulation, students still need to make all measurements and calculations in order to determine the values. An extra feature of this simulation is that time may be accelerated in order to speed up observations of motion, as the terminal velocity of the miniscule droplets is very slow. Modes There are two modes for running the experiment. Using the correct, real world value of e (the fundamental charge), students may use either beads (microspheres of fixed mass) or oil droplets (mass must be determined by finding the terminal velocity) to determine the number of excess electrons on the particle. When the mode is switched to ‘Unknown value of e’, the program uses an alternate value for the fundamental charge. Students use beads or oil drops to determine that charge, just as Millikan and Fletcher did. All unknown values of e are close to the real value. These values are known to the author, but not made public – so nobody can cheat. Students using this for a lab must collect enough evidence to make a valid argument, and the instructor will have to gauge the result by the quality of the data and presentation, not by accuracy. Further instructions are provided in the HELP file in the program itself and copied below for review. Using the Simulator Begin by launching an oil droplet using the New Droplet button. Unlike the original experiment, this simulation launches only one droplet at a time. If Oil Droplets is selected, the mass of the droplet is random. If Bead is selected, the droplet has a fixed mass of 1x10-15 kg. The plates are initially set at a separation distance of 2.5 cm. The spacing can be adjusted using the buttons below the lower plate. The scale (marked in 1 mm gradations) can also be turned on or off as necessary. Turn the power on by clicking the On button. Use the coarse and fine voltage adjustments to adjust the potential so that the droplet remains motionless. The polarity switch reverses the polarity of the plates. When tuning the voltage, the Time Speed slider may be used to speed up the apparent rate of motion so that the voltage can be set more precisely. Once the potential required to support the droplet is determined, cut the power and use the built-in stopwatch to time the rate of descent (each mark on the scale is 1 mm). Since the droplets are very small, they reach terminal velocity almost instantly. Using the values for potential and terminal velocity, the charge can be determined. Determining the charge on an electron Given the density of the oil droplet (in this case, 900 kg/m3), the mass of the droplet can be found using the equation 4 𝑚 = 𝜋𝑟 3 𝜌 3 The radius, in turn, can be found if the density and viscosity of air are known, using the equation: 9 𝑛𝑣0 𝑟= √ 2 𝑔(𝜌 − 𝑝0 ) Combining these two, we get the equation: 3 𝑚= 4 9 𝑛𝑣0 𝜋𝜌 ( √ ) 3 2 𝑔(𝜌 − 𝑝0 ) Inserting the density of the oil, the density of the air and the viscosity of the air, this equation simplifies to the following: 𝑚 = 3.32477 × 10 −9 3 (𝑣02 ) Given the terminal velocity v0, the mass can easily be calculated. When the droplet is suspended motionless, the electric and gravitational forces are balanced: 𝐹𝑒 = 𝐹𝑔 𝐸𝑞 = 𝑚𝑔 The charge on the electron can be determined using: 𝑞= 𝑚𝑔 𝐸 or 𝑞 = 𝑚𝑔𝑑 𝑉

















