P90 Patient registry experience (2006
advertisement

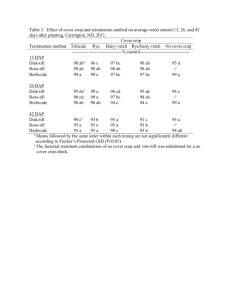
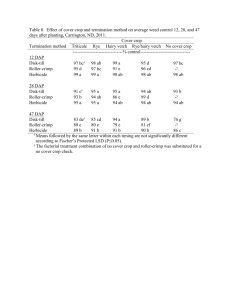
P90 PATIENT REGISTRY EXPERIENCE (2006-11): DAPTOMYCIN IN THE TREATMENT OF GRAM-POSITIVE INFECTIONS Gonzalez-Ruiz, A1, Andrews, C2, Jones, A3, Milne, D2 on behalf of the UK EU-CORE group ¹Darent Valley Hospital, Dartford, UK, ²Novartis Pharmaceuticals, Frimley, UK, ³MSC Ltd, North Yorkshire, UK INTRODUCTION: Daptomycin (DAP), a cyclic lipopeptide antibiotic with Gram-positive activity, was licensed in the UK for the treatment of complicated skin and soft tissue infections (cSSTIs) and right-sided infective endocarditis with or without Staphylococcus aureus bacteraemia (SAB). Data on the use of DAP has been collected in the patient registry European Cubicin® Outcomes Registry and Experience (EU-CORESM). AIMS/OBJECTIVES: To present data for the first 5.5 years of daptomycin (DAP) use for the treatment of Gram-positive infections since marketing authorisation. METHODS: Data from all participating UK institutions (13) were collected from the retrospective multicentre, non-interventional registry (EU-CORE) including demographics, antibiotic usage, microbiological and clinical outcomes and adverse events from patients treated with DAP between Jan 2006 and June 2011. Patients were categorised by type of infection. All pts included in the registry received >1 dose of DAP with 30 day follow up data available. Outcomes were assessed by investigators as cured, improved, failure or nonevaluable. All adverse events were recorded. RESULTS: In total, 590 patients were entered into the registry. All patients were included in the efficacy and safety population. Primary infections included SSTI (40%), bacteraemia (19%), endocarditis (13%), foreign body/prosthetic (10%) and osteomyelitis (7%). 525 (89%) of patients had underlying disease of which 78 (13%) had renal disease. Of the127 patients with a primary diagnosis of bacteraemia, 61 were catheter-related and 66 non-catheter-related and 65 (51%) of patients had received prior antibiotics within 48 hours of DAP (37 had received β-lactams with or without other antibiotics). Overall, 75% (444/590) of clinical outcomes were success (defined as ‘cured or improved’) 9% (51/590) failure and 16% (95/590) non-evaluable and for patients with bacteraemia 65% (82/127) were success, failure in 14% (18/127) and non-evaluable in 21% (27/127) of pts. Mean time to improvement was 4.8 days. Mean duration of therapy was 14.2 days overall and 8.3 days in patients with bacteraemia. 359 patients had a pathogen isolated, of which the most frequent were: S. aureus (44%, 159/359 overall and 26%, 33/127 bacteraemia) and coagulase-negative staphylococci (17%, 61/359 overall and 28%, 35/127 bacteraemia). Adverse events possibly related to study drug were experienced by 37/590 (6%) of pts. Blood creatine phosphokinase increased in 6 (1%) patients, rhabdomyolysis was reported for 2 (0.3%) patients and myalgia for 1 (0.2%). CONCLUSIONS: DAP was used in the UK to treat a wide range of Gram-positive infections in patients with multiple comorbidities. DAP has an important role as therapy for Gram-positive bacteraemia, and may also be effective when other antibiotics have failed. These data may support the earlier use of DAP in patients with suspected Gram-positive bacteraemia.