Review of Main Ideas
advertisement
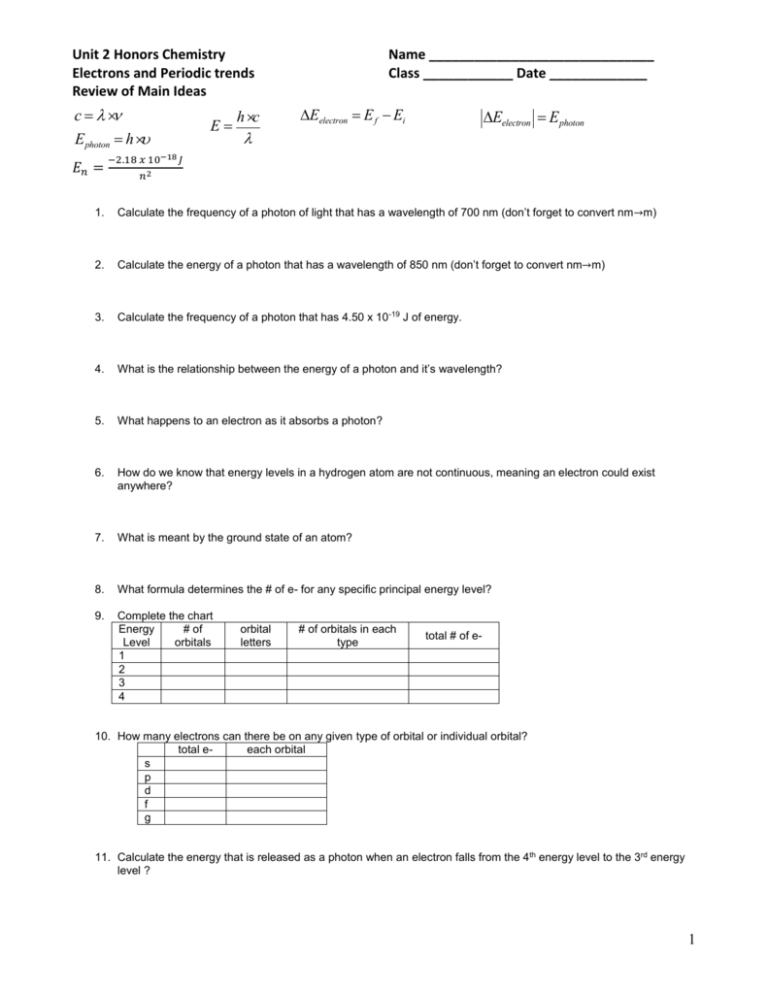
Unit 2 Honors Chemistry Electrons and Periodic trends Review of Main Ideas c = l ×n E photon = h × u 𝐸𝑛 = E= h×c Name ______________________________ Class ____________ Date _____________ DEelectron = E f - Ei DEelectron = E photon l −2.18 𝑥 10−18 𝐽 𝑛2 1. Calculate the frequency of a photon of light that has a wavelength of 700 nm (don’t forget to convert nm→m) 2. Calculate the energy of a photon that has a wavelength of 850 nm (don’t forget to convert nm→m) 3. Calculate the frequency of a photon that has 4.50 x 10-19 J of energy. 4. What is the relationship between the energy of a photon and it’s wavelength? 5. What happens to an electron as it absorbs a photon? 6. How do we know that energy levels in a hydrogen atom are not continuous, meaning an electron could exist anywhere? 7. What is meant by the ground state of an atom? 8. What formula determines the # of e- for any specific principal energy level? 9. Complete the chart Energy # of Level orbitals 1 2 3 4 orbital letters # of orbitals in each type total # of e- 10. How many electrons can there be on any given type of orbital or individual orbital? total eeach orbital s p d f g 11. Calculate the energy that is released as a photon when an electron falls from the 4th energy level to the 3rd energy level ? 1 12. Write the electron configuration for a Sc2+ ion. 13. Identify the elements based on their configurations: a. [Ar]4s13d10 b. [Kr]5s14d5 c. [Xe]7s25f4 14. How do atoms become ions, what are positive ions called, what are negative ions called? 15. What type of ion is formed from each atom? a. Mg b. O c. Li d. Al e. N 16. What information does the orbital filling diagram tell you that an electron configuration does not? 17. When filling a p orbital, why do you separate the electrons into different orbitals, before pairing them up. What is the name of this rule? 18. What information does a Lewis dot diagram tell you? 19. What do the elements in the same period have in common? 20. What do elements in the same group have in common? 21. Write the lewis dot diagram for the folowing atoms: a. Sr e. Ne b. Zn f. H c. C g. V d. P h. U 22. How does Bohr's "electron orbits" differ from the "electron orbitals"? 23. Why do some properties of elements repeat in periodic patterns? 24. What types of ions do metals form? 25. What types of ions do nonmetals form? 26. For each of the following properties, indicate whether fluorine or iodine has a larger value: a) electronegativity b) electron affinity c) atomic radius d) ionization energy. 2 27. Explain why radius of an atom cannot be measured directly. 28. Which elements are characterized as having their d orbitals fill with electrons as you move left to right across a period? 29. What is the difference between a core (a.k.a. shielding) electron and a valence electron? 30. Explain why it’s harder to remove a shielding electron than a valence electron from an atom? 31. An element forms an cation when ionized. On what side of the periodic table is the element located? Explain. 32. What is the Periodic Law? 33. What is Hund’s Rule? 34. What is the Aufbau Principle? 35. What is the Pauli Exclusion Principle? 36. Explain why each successive ionization of an electron requires a greater amount of energy. 37. Na+ and Mg2+ ions each have ten electrons surrounding their nuclei. Which ion would you expect to have the larger radius? Explain. 38. Draw the Lewis dot diagram, and electron configuration for the following elements. Lewis-dot Electron configuration (use noble gas shortcut) Sybmol diagram Ne Cu S2Pu Ca2+ 39. How does the atomic radius change from left to right across a period. Explain why? 40. How does the atomic radius change from top to bottom for a group. Explain why? 3 41. How does the ionization energy change from left to right across a period. Explain why? 42. How does the ionization energy change from top to bottom for a group. Explain why? 43. Why do large atoms have small ionization energies? 44. Why are metal ions smaller than their metal atoms? 45. Why are nonmetal ions larger than their nonmetal atom? 46. Why does F have a higher electronegativity than Li? Be specific? 47. What electron configuration has the greatest stability? 48. Order the following groups from smallest to largest radii. a. I, Ba, Cs, Xe b. Kr, Br, Sr, Se c. Li, K, Fr, Cs 49. Order the following groups from highest to lowest ionization energy. a) Na, Mg, Ca b) Br, F, Se c) K, Sr, Ba 50. Describe effective nuclear charge (Zeff). 51. Calculate the Zeff for Ca and Br. Can Zeff be used to explain atomic radius trends? Explain. 52. What is ionization energy? 53. Why is the 3rd ionization energy of Mg so much larger than it’s 2 nd IE? 54. Why is the ionization energy for Al less than Mg? 55. In which group are the three species isoelectronic (all have same # of e-)? (A) S2-, K+, Ca2+ (B) Sc, Ti, V2+ (C) O2-, S2-, CI(D) Mg2+, Ca2+, Sr2+ 56. The ionization energies for element X are listed in the table below. On the basis of the data, element X is most likely Ionization Energies for element X (kJ mol-1) First Second Third Fourth Fifth 580 1,815 2,740 11,600 14,800 (A) Na (B) Mg (C) Al (D) Si 57. Which property generally decreases across the periodic table from sodium to chlorine? (A) 1st ionization energy (B) Atomic mass (C) Ionic radius (D) Atomic radius 4 5 6








