all12141-sup-0001-Data_S1
advertisement

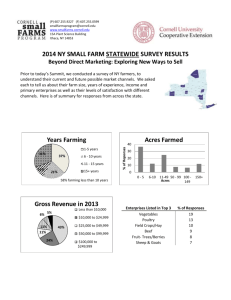
Asthma and allergies: is the farming environment (still) protective in Poland? The GABRIEL Advanced Studies. Supporting materials Stephanie J MacNeill1, Barbara Sozanska2, Hanna Danielewicz2, Anna Debinska2, Aleksandra Kosmeda2, Andrzej Boznanski2, Sabina Illi3, Martin Depner3, Christine Strunz-Lehner3, Marco Waser4,5, Gisela Büchele6, Elisabeth Horak7, Jon Genuneit6, Dick Heederik8, Charlotte Braun-Fahrländer4,5, Erika von Mutius3 and Paul Cullinan1 and the GABRIELA study group 1 Department of Occupational and Environmental Medicine, National Heart and Lung Institute, Imperial College London, London, UK 2 Department of Paediatrics, Allergology and Cardiology, Wrocław Medical University, Wrocław, Poland 3 Asthma and Allergy Research Group, University Children’s Hospital, Munich, Germany 4 Swiss Tropical and Public Health Institute, Basel, Switzerland 5 University of Basel, Basel, Switzerland 6 Institute of Epidemiology and Medical Biometry, University of Ulm, Ulm, Germany 7 Department of Paediatrics and Adolescents, Division of Cardiology and Pulmonology, Innsbruck Medical University, Innsbruck, Austria 8 Division of Environmental Epidemiology, Institute for Risk Assessment Sciences, Utrecht University, Utrecht, The Netherlands The members of the GABRIELA study group are in alphabetical order: Silvia Apprich PhDG, Andrzej Boznanski MD, PhDJ, Charlotte Braun-Fahrländer MDD,E, Gisela Büchele PhDC, William Cookson MD, DPhilA, Paul Cullinan MDA, Hanna Danielewicz MDJ, Anna DębińskaJ, Martin Depner PhDB, Markus Ege MDB, Urs Frey MD, PhDQ , Oliver Fuchs MDK, Jon Genuneit MDC, Dick Heederik PhDF, Elisabeth Horak MDL, Anne Hyvärinen PhDH, Sabina Illi PhDB, Michael Kabesch MDM, Katalin KovacsL, Aleksandra Kosmęda PhDJ, Wolfgang Kneifel PhDG, Philipp Latzin MD, PhDK, Roger Lauener MDO , Georg Loss MScD,E, Stephanie MacNeill PhDA, Bernhard Morass MDL, Anne-Cécile Normand PhDP, Ilka Noss PhDF, Renaud Piarroux MD, PhDP, Helena Rintala PhDH, Mascha K Rochat MDB, Nikolaos SitaridisC, Barbara Sozanska MDJ, David Strachan MDN, Christine Strunz-Lehner MPHB, Bertrand Sudre MD, PhDI, Erika von Mutius MD, MScB, Marco Waser PhDD,E, Juliane Weber MDB, Inge Wouters PhDF A. Imperial College London, National Heart and Lung Institute, South Kensington Campus, London SW7 2AZ, United Kingdom B. LMU Munich, University Children’s Hospital, Lindwurmstrasse 4, D 80337, Munich, Germany C. Ulm University, Institute of Epidemiology and Medical Biometry, Helmholtzstraße 22, D-89081 Ulm, Germany D. Swiss Tropical and Public Health Institute, Socinstr. 57, P.O. Box, 4002 Basel, Switzerland E. University of Basel, Petersplatz 1, 4003 Basel, Switzerland F. Utrecht University, Institute for Risk Assessment Sciences (IRAS), Division of Environmental Epidemiology, PO Box 80178, 3508TD, Utrecht, The Netherlands G. BOKU Vienna, University of Natural Resources and Life Sciences, Department of Food Science and Technology, Muthgasse 18, A-1190 Vienna, Austria H. THL Kuopio, National Institute for Health and Welfare, PL 95 70701 Kuopio, Finland I. Université de Franche-Comté, UMR 6249 Chrono-Environnement, Département de Parasitologie/Mycologie, UFR SMP, 19 rue A. Paré, 25000 Besançon, France J. Wroclaw Medical University, 1st Department of Paediatrics, Allergology and Cardiology, ul. J.M. Hoene-Wronskiego 13C, 53-376 Wroclaw, Poland K. Division of Pulmonology, Department of Paediatrics, Bern University Hospital, Bern, Switzerland L. Department of Pediatrics and Adolescents, Division of Cardiology and Pulmonology, Innsbruck Medical University, Anichstr. 35, A-6020, Innsbruck, Austria M. Hannover Medical School, Clinic for Paediatric Pneumology and Neonatology, Carl-Neuberg-Str. 1, D-30625 Hannover, Germany N. St George’s, University of London, Cranmer Terrace, London SW17 0RE, United 68 Kingdom O. High Mountain Hospital Davos, Herman-Burchard-Str. 1, CH-7265 DavosWolfgang, Switzerland P. Department of Parasitology and Mycology, Hôpital de la Timone, Assistance Publique-Hôpitaux de Marseille, 13385 Marseille, France Q. University Children’s Hospital (UKBB), Spitalstrasse 33, 4056 Basel, Switzerland Corresponding author and address for reprint requests: SJ MacNeill, PhD Department of Occupational and Environmental Medicine National Heart and Lung Institute, Imperial College London 1b Manresa Road London SW3 6LR UK Telephone: +44-20-7351-8397 Fax: +44-20-7351-8336 Email: s.macneill@imperial.ac.uk Statistical methods In the analysis of the Phase I findings, all data are presented as frequencies and proportions. The chi-squared test was used to test for differences between farm and non-farm children. Weighted statistical methods taking into account the stratified sampling design were used in analyses of the Phase II population. Fixed, prior weights were calculated as the ratio of the “eligible” to “selected” children per stratum as identified in Phase I; thus, the prevalences of demographic characteristics, specific farming exposures and outcomes are presented as weighted prevalences. Univariate comparisons between farm and non-farm children were performed using Pearson’s chi-squared test with the Rao and Scott correction (1). We used adjusted logistic regression models to assess the effects of specific farm characteristics identified in the Phase I questionnaire on childhood asthma, hay fever and atopic sensitisation among village children. Models for atopic sensitisation were weighted to account for Phase II survey sampling. For each outcome a separate model adjusting for potential confounders was fitted for each farming exposure and all farming exposures found to have a statistically significant association with the outcome were added to a final model. Potential confounders used in these models were those variables significantly associated with farming among villagers in the Phase I population for childhood asthma and hay fever and in the Phase II population for atopic sensitisation. To assess the effects of early-life specific childhood farm exposures identified in the Phase II questionnaire on atopic sensitisation, we used weighted logistic regression models adjusting for both farming and potential confounders. A separate model was fitted for each specific exposure. Potential confounders used in these models were those variables significantly associated with farming in the Phase II population through univariate weighted logistic regression models. A stratified analysis was performed to determine whether the impact of childhood exposure to farm milk differed by whether or not the child had any exposure to cows, pigs, horse or sheep between pregnancy and age three years. In order to detect the specific childhood farm exposure variables that could explain the overall farm effect, weighted, step-wise, forward-fitting logistic regression models were constructed. For each measure of atopic sensitisation, exposures eligible for inclusion in the model were those significantly associated with the outcome and whose inclusion in the model altered the adjusted odds ratio (aOR) for farming by at least 10%. To assess the impact that the inclusion of each childhood exposure had on farming, models with and without the exposure – using the same sample in both models – were compared. The significant exposure resulting in the largest change in the farming aOR was included first and the step-wise fitting procedure was repeated with the remaining exposure variables. In a backwards step, exposures were removed from the model if they were no longer statistically significant. The step-wise procedure ended when none of the remaining exposures fulfilled the original entry criteria. To determine whether the results of the resulting final models were sensitive to the sampling weights, models were refitted with weights equal to the ratio of the “eligible” population to those having complete data for the variables in the respective model. To assess whether the number of different exposures – a proxy measure of diversity – was protective, regression models adjusting for farming, the number of farm exposures the child was exposed to and potential confounders were constructed. The weighted distribution of the number of different early life exposures was distributed into approximate quartiles (no exposure, 1 exposure, 2-4 exposures and 5 or more exposures) and assessed as a categorical variable. The degree of correlation between specific farm exposures identified in Phase II was explored using Kendall’s τb statistic for all possible pair-wise comparisons. This descriptive analysis did not adjust for the stratified sampling design. We assessed whether early-life exposures were associated with atopy in the village farm substrata of the Phase II population using weighted forward-fitting stepwise models. These models adjusted for the same potential confounders as those for the full Phase II population which included farming as a covariate. Exposures eligible for inclusion in the models were those significantly associated with the outcome. The exposure resulting in the smallest t-statistic with p-value<0.05 was added first. The second step functioned in the same manner and the procedure finished when none of the remaining variables were significantly associated with atopy in the adjusted model. Statistical analyses were performed in STATA v11 (StataCorp, USA). Due to the exploratory nature of the analyses, corrections for multiple significance testing were not performed. References (1) Rao JNK, Scott AJ. On Chi-Squared Tests for Multiway Contingency-Tables with Cell Proportions Estimated from Survey Data. Annals of Statistics 1984;12(1):46-60.