Phase Changes and Heating Curves
advertisement
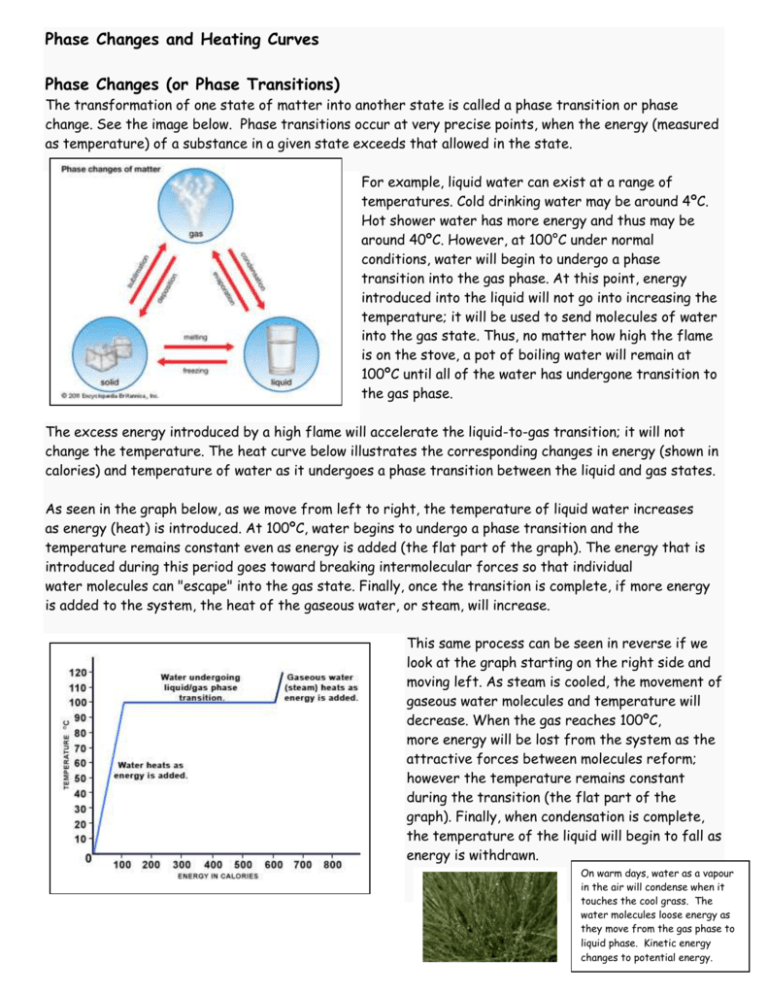
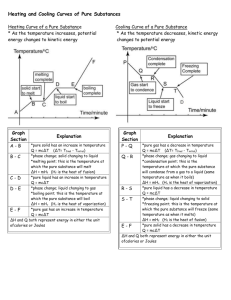
Phase Changes and Heating Curves Phase Changes (or Phase Transitions) The transformation of one state of matter into another state is called a phase transition or phase change. See the image below. Phase transitions occur at very precise points, when the energy (measured as temperature) of a substance in a given state exceeds that allowed in the state. For example, liquid water can exist at a range of temperatures. Cold drinking water may be around 4ºC. Hot shower water has more energy and thus may be around 40ºC. However, at 100°C under normal conditions, water will begin to undergo a phase transition into the gas phase. At this point, energy introduced into the liquid will not go into increasing the temperature; it will be used to send molecules of water into the gas state. Thus, no matter how high the flame is on the stove, a pot of boiling water will remain at 100ºC until all of the water has undergone transition to the gas phase. The excess energy introduced by a high flame will accelerate the liquid-to-gas transition; it will not change the temperature. The heat curve below illustrates the corresponding changes in energy (shown in calories) and temperature of water as it undergoes a phase transition between the liquid and gas states. As seen in the graph below, as we move from left to right, the temperature of liquid water increases as energy (heat) is introduced. At 100ºC, water begins to undergo a phase transition and the temperature remains constant even as energy is added (the flat part of the graph). The energy that is introduced during this period goes toward breaking intermolecular forces so that individual water molecules can "escape" into the gas state. Finally, once the transition is complete, if more energy is added to the system, the heat of the gaseous water, or steam, will increase. This same process can be seen in reverse if we look at the graph starting on the right side and moving left. As steam is cooled, the movement of gaseous water molecules and temperature will decrease. When the gas reaches 100ºC, more energy will be lost from the system as the attractive forces between molecules reform; however the temperature remains constant during the transition (the flat part of the graph). Finally, when condensation is complete, the temperature of the liquid will begin to fall as energy is withdrawn. On warm days, water as a vapour in the air will condense when it touches the cool grass. The water molecules loose energy as they move from the gas phase to liquid phase. Kinetic energy changes to potential energy. Phase transitions are an important part of the world around us. For example, the energy withdrawn when perspiration evaporates from the surface of your skin allows your body to correctly regulate its temperature during hot days. Phase transitions play an important part in geology, influencing mineral formation and possibly even earthquakes. And who can ignore the phase transition that occurs at about -3ºC, when cream, perhaps with a few strawberries or chocolate chunks, begins to form solid ice cream. Now we understand what is happening in a pot of boiling water. The energy (heat) introduced at the bottom of the pot causes a localized phase transition of liquid water to the gaseous state. Because gases are less dense than liquids, these localized phase transitions form pockets (or bubbles) of gas, which rise to the surface of the pot and burst. But nature is often more magical than our imaginations. Despite all that we know about the states of matter and phase transitions, we still cannot predict where the individual bubbles will form in a pot of boiling water. Difference between evaporation and boiling: Evaporation Boiling Vapour pressure measures the tendency for the molecules to escape from liquid to the gas phase. At lower temperatures the vapour pressure of a liquid is much lower than the pressure on the surface of the liquid. When the temperature of the liquid is gradually increased, its vapour pressure also increases. Ultimately a stage is reached when the vapour pressure of the liquid equals the pressure of the air above it. At this point, molecules and vapours formed within the liquid can easily rise through the liquid in the form of bubbles and escape into the air. This phenomenon is known as boiling and the temperature at which this occurs is known as boiling point. Boiling point is the temperature at which the vapour pressure of a liquid becomes equal to the atmospheric pressure. The boiling point, therefore, depends upon the atmospheric pressure and it changes with the change in the pressure above the liquid. As the atmospheric pressure increases, it is necessary to heat the liquid to a higher temperature to make its vapour pressure equal to atmospheric pressure. At high altitudes, having lower atmospheric pressure, water boils at a much lower temperature than in the plains where atmospheric pressure is higher. Once the boiling starts, the temperature of the liquid remains constant, until the whole of the liquid has vaporized, even though heating is continued. Heating Curve of a Pure Substance * As the temperature increases, potential energy changes to kinetic energy * Endothermic process; energy is absorbed Cooling Curve of a Pure Substance * As the temperature decreases, kinetic energy changes to potential energy * Exothermic process; energy is released s Graph Explanation Section *pure solid has an increase in temperature A-B Q = mc∆T (∆T= Tfinal - Tinitial) *phase change; solid changing to liquid B-C *melting point; this is the temperature at which the pure substance will melt ∆H = mHf (Hf is the heat of fusion) *pure liquid has an increase in temperature C-D Q = mc∆T *phase change; liquid changing to gas D-E *boiling point; this is the temperature at which the pure substance will boil ∆H = mHv (Hv is the heat of vaporization) *pure gas has an increase in temperature E-F Q = mc∆T ∆H and Q both represent energy in either the unit of calories or Joules HEAT CAPACITY (c) OF WATER liquid: 4.184 J/g °C solid: 2.03 J/g °C gas: 2.01 J/g °C VALUES FOR WATER latent heat of fusion Hf: 334 J/g latent heat of vaporization Hv: 2260 J/g Graph Explanation Section *pure gas has a decrease in P-Q temperature Q = mc∆T (∆T= Tfinal - Tinitial) *phase change; gas changing to liquid Q-R *condensation point; this is the temperature at which the pure substance will condense from a gas to a liquid (same temperature as when it boils) ∆H = mHv (Hv is the heat of vaporization) *pure liquid has a decrease in R-S temperature Q = mc∆T *phase change; liquid changing to solid S-T *freezing point; this is the temperature at which the pure substance will freeze (same temperature as when it melts) ∆H = mHf (Hf is the heat of fusion) *pure solid has a decrease in T-D temperature Q = mc∆T ∆H and Q both represent energy in either the unit of calories or Joules EXAMPLE #1: Calculate the amount of heat required to completely convert 50 g of ice at -10 ºC to steam at 120 ºC. Sketch a graph of this change, provide an appropriate title and label both the x and y axis with the correct units. SOLUTION: This is a heating curve. Heat is taken up in five stages. The heat taken up in the complete process is the sum of the heat taken up in each stage. Endothermic process. 1. (A–B) Heat taken up as ice heats from -10 ºC to the melting point, 0 ºC. Q = mc∆T (A–B) Q = mass of water x specific heat ice x temperature change Q = 50 g x 2.03 J/gºC x 10 ºC Q = 1015 J 2. (B-C) Heat taken up to ∆H = mHf (B-C) convert ice to water (melting). ∆H = mass of water x latent heat of fusion Phase change occurring. ∆H = 50 g x 334 J/g ∆H = 16700 J 3. (C-D) Heat taken up by liquid water from 0 ºC to the boiling point, 100 ºC. Q = mc∆T (C-D) Q = mass of water x specific heat liquid water x temp. change Q = 50 g x 4.184 J/gºC x 100 ºC Q = 20900 J 4. (D-E) Heat taken up vaporizing the water (boiling). Phase change occurring. ∆H = mHV (D-E) ∆H = mass of water x latent heat of vaporization ∆H = 50 g x 2260 J/g ∆H = 113000 J 5. (E-F) Heat taken up as the Q = mc∆T (E-F) NOTE: water vapour = steam steam (gas) heats from 100 ºC Q = mass of water x specific heat water vapour x temp. change to 120 ºC. Q = 50 g x 2.01 J/gºC x 20 ºC Q = 2010 J The sum of these is: 1015 J + 16700 J + 20900 J+ 113000 J + 2010 J = 153625 J approximately 154 kJ NOTE: 1000 kJ = 1 J EXAMPLE #2: Calculate the amount of heat lost when 13 g of water vapour (steam) at 103 ºC changes to ice at -4 ºC. Sketch a graph of this change, provide an appropriate title and label both the x and y axis with the correct units. SOLUTION: This is a cooling curve. Heat is lost in five stages. The heat released in the complete process is the sum of the heat lost in each stage. Exothermic process so each calculation in the five steps results in a negative value - indicating the loss of heat. 1. (A–B) Gas (or steam) loses Q = mc∆T (A–B) heat as it cools from 103 ºC to Q = mass of water x specific heat steam x temperature change 100 ºC. Q = 13 g x 2.01 J/gºC x (-3 ºC) Q = -78 J 2. (B-C) Heat lost as gas condenses to liquid. Phase change occurring. ∆H = mHv (B-C) ∆H = mass of water x latent heat of vapourization ∆H = 13 g x (- 2260 J/g) ∆H = -29380 J 3. (C-D) Heat lost as the Q = mc∆T (C-D) water cools from 100 ºC to the Q = mass of water x specific heat liquid water x temp. change freezing point of 0 ºC. Q = 13 g x 4.184 J/gºC x (-100 ºC) Q = -5439 J 4. (D-E) Heat lost as water freezes. Phase change occurring ∆H = mHf (D-E) ∆H = mass of water x latent heat of fusion ∆H = 13 g x (-334 J/g) ∆H = -4342 J 5. (E-F) Heat lost as ice cools from 0 ºC to -4 ºC. Q = mc∆T (E-F) Q = mass of water x specific heat ice x temp. change Q = 13 g x 2.03 J/gºC x (-4 ºC) Q = -106 J The sum of these is: (-78 J) + (-29380 J) + (-5439 J) + (-4342 J) + (-106 J) = 39345 J approximately 39 kJ