Quiz: Unit 6A—Chemical Reactions
advertisement

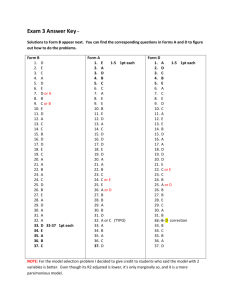
Name:_____________________________________________Period______Date_________________ Quiz: Unit 6A—Chemical Reactions MnO2 1. Label the parts of this equation (4pts): A. Catalyst B. Coefficient 2H2O2 2H2O + O2 C. Superscript D. Subscript E. Yields or Produces 2. Which greenhouse gas is produced from the combustion of fossil fuels (hint: also the gas released when hydrocarbons are burned) (1pt):___________________________________ Label which side of the equation is the “product(s)” and the “reactant(s)” in the following: 3. __________________Ca +2HCl ---> CaCl2 + H2________________(1pt) 4. __________________2KClO3 ----> 2KCl + 3O2________________(1pt) 5. Which of the above reactions , #4 or #5, is endothermic?_____________(1pt) The five reaction types are: synthesis, decomposition, single and double replacement, combustion 6. Which of the five types of reactions is the equation in #3__________________________(1pt) 7. Which of the five types of reactions is the equation in #4__________________________(1pt) 8. When a firecracker ignites and explodes, it give-off light and heat. Is that endothermic or exothermic?________________________________(1pt) 9. Circle all of the observations below that signify that a chemical reaction has taken place: Freezing Cut in half Release of a gas Sublimation Melting Absorb heat Formation of a precipitate Release light Change in color Evaporation 10. Circle which of these changes in rock is a physical change? (1pt) An ice wedge falling from a cliff and shattering a piece of sandstone Iron in rock combining with oxygen to produce the red rust color in a hematite rock Carbonic acid dissolving limestone in Austin to form caves Acid rain falling in New York and eroding statues made of marble rock 11. Circle which of the following are chemical changes Salt dissolving in water Water boiling Chocolate melting fireworks exploding the flame of a candle biting an apple decaying wood digesting an apple The five reaction types are: synthesis, decomposition, single and double displacement, combustion. Identify the correct reaction occurring below. 12. CaO + H2O Ca(OH)2 13. Cu(ClO3)2 14. C2H6 + 3O2 2CO2 + 3H2O 15. CaCO3 + 2HCl CaCl2 + H2CO3 16. Br2 + 2CuI 2CuBr + I2 HEAT > 3O2 + CuCl2 TYPE OF RXN:_____________________________(1pt) TYPE OF RXN:_____________________________(1pt) TYPE OF RXN :_____________________________(1pt) TYPE OF RXN:____________________________(1pt) TYPE OF RXN:____________________________(1pt) Mg + HCl MgCl2 + H2 17. When the above equation is balanced, circle the correct coefficient for magnesium chloride A. 1 B. 4 C. 0 D. 2 (1pt) 18. What is the coefficient for H2O when the above equation is balanced? K + H2O KOH + H2 A. 3 B. 2 C. 4 D. 1 (1pt) 19. Chemical equations must be balanced to satisfy the… (1pt) a. Law of Definite Proportions b. Law of Conservation of Mass c. Law of Multiple Proportions d. Principle of Mendeleev W20. Which chemical equation supports the law of conservation of mass? (1pt) a. 2H2O(l) + H2(g) O2(g) b. Zn(s) + HCl(aq) ZnCl2(aq)+ H2(g) c. Al4C3(s) + H2O(l) CH4(aq)+ Al(OH)3(s) d. CH4(g) + 2O2(g) CO2(g)+ 2H2O(g) **30 points total. Count all point awarded and write as a fraction over 30 at top of first page.