Compounds - Fulton County Schools
advertisement

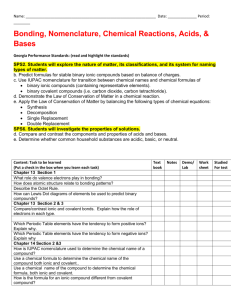
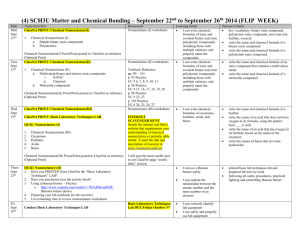
Department: ___Science_______________ Teacher: ___Davis_________________ Course: ____Honors Chemistry______________ Topic / Theme: ___Types of Matter and Compound Nomenclature_______________________ ____ Duration:_____~ 2 ½ weeks_____________________ Essential Question: Key Questions: Essential Vocabulary: How can matter be classified and named? What is the definition of matter? What are the four phases of matter? What is the difference in a physical and chemical property? What is the difference between a physical and chemical change? What is the difference in an intensive and extensive property? What is the difference in an element, compound, and mixture? How is IUPAC nomenclature used to determine the names and formulas of covalent compounds, ionic compounds, and acids? matter, solid, liquid, gas, plasma, physical property, physical change, chemical property, chemical change, intensive property, extensive property, density, element, compound, mixture, heterogeneous, homogeneous, ionic compound, covalent compound, acid, binary, tertiary, IUPAC nomenclature, empirical formula, molecular formula, superscript, subscript Standards (List the primary standard and the key verbs from the elements) Higher-Order Student Engagement (Specific activities/tasks that will actively engage students in higher order learning) SC1 Students will analyze the nature of matter and its classifications. b. Identify substances based on chemical and physical properties. c. Predict formulas for stable ionic compounds (binary and tertiary) based on balance of charges. d. Use IUPAC nomenclature for both chemical names and formulas: • Ionic compounds (Binary and tertiary) • Covalent compounds (Binary and tertiary) Call on specific students with content specific questions every day. Nightly Homework Problems. Unit Review Homework/Study Guide. Laboratory Activities. Unit Test Assessment of Learning Goals : Formative and Summative Laboratories (honors chemistry tasks require students to apply knowledge to solve problems) (How do you know if your students have learned?) Call on specific students with content specific questions every day. Nightly Homework Problems. Polyatomic Ion Quizzes Compound Quizzes Unit Review Homework/Study Guide. Laboratory Activities. Unit Test Thickness of Aluminum Foil Lab Separation of Mixture Lab We empower students to discover, think, and succeed. GPS Standards (Optional) SC1 Students will analyze the nature of matter and its classifications. b. Identify substances based on chemical and physical properties. c. Predict formulas for stable ionic compounds (binary and tertiary) based on balance of charges. d. Use IUPAC nomenclature for both chemical names and formulas: • Ionic compounds (Binary and tertiary) • Covalent compounds (Binary and tertiary) We empower students to discover, think, and succeed.