Lab Ionic vs Covalent
advertisement

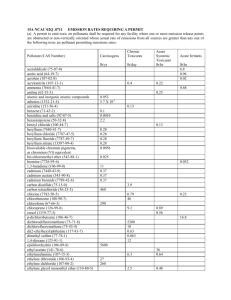
Lab: Ionic vs Covalent Name: For this lab you will make your hypothesis, test your hypothesis, record data, analysis the data. Upon completion of the lab you must type this lab up as a formal lab report and bring the final lab report printed out and turned in by the due date April 11th. You may turn the report in earlier than the due date. You must have the lab report already printed before coming to class to turn it in. This rough draft format will be on the class website for you to go and type the information in from your rough draft. Purpose: Hypothesis: (Based on the properties of ionic and covalent compounds make a hypothesis as to which type of bond each compound will have): Compound 1.Gelatin 2.Sugar 3.Sodium chloride 4.Sodium bicarbonate Hypothesis Compound 5. Potassium chloride 6. Borax 7. Starch 8. Copper (II) chloride Hypothesis Materials: Compound Samples #1-8 in petri dishes, Compound Samples #1-8 in solutions, googles, aprons, magnifying glass, pipet, dropper plate, lab sheet Procedure: 1. 2. 3. 4. Obtain your googles and apron. Go to your assigned lab station Observe and write a description of the compounds in your data table. Next observe the solutions in the beakers. If the compound dissolves write SOLUBLE in the data table under “Solubility”. If it does not dissolve, write INSOLUBLE under “Solubility” column. 5. Using the pipet fill the depression in dropper plate and test the solution with the conductivity tester according to the teachers instructions. ONLY the copper probe wires should come in contact with the solution. DO NOT allow the other parts of the conductivity probe to come in contact with the solutions or get wet in any way. Record the results of your conductivity test in the data table. 6. After testing the first solution use a wet paper towel to wipe the copper probes clean before testing the next solution. Repeat steps 5 and 6 for each of the other solutions. Recording data on the data table as you proceed. 7. Once you have tested all solutions and cleaned the probe, dump solutions from the dropper plate into the waste container at each table. Clean and dry the dropper plate. Wash your hands and return the googles and aprons to their storage area. Return to the room and answer the follow up questions. Take work home and type up your lab report. DATA TABLE: Compound Gelatin Description Solubility Conductivity Bond Sugar Salt Sodium Bicarbonate Potassium Chloride Borax Starch Copper (II) Chloride Analysis Questions: Write your answers in complete sentences. 1. Some characteristics of many ionic compounds include solubility in water and the ability to conduct electricity. On the basis of these two properties, which compounds appear to have ionic bonds (please indicate in last column “Type of Bond” in data chart)? 2. Water solutions of covalent compounds do not conduct electricity. Based on this property, which compounds that you tested would you classify as covalent compounds (please indicate in last column “Type of Bond” in data chart)? 3. Explain how ionic and covalent compounds are different. USE EVIDENCE FROM THE LAB. 4. Did all of the compounds in solution that conducted electricity show the same amount of conductivity? How can you tell (from the lab)? 5. Solid sodium chloride does not conduct electricity. Why do you think dissolving sodium chloride in water allows the salt to conduct electricity? Use your knowledge of compounds and information from the “How it Works: Salt” video to answer this question.