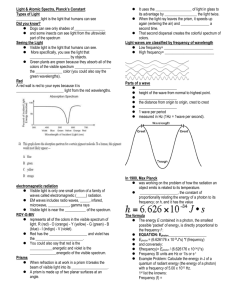
Light as a particle: During the 1800`s the concept that light was a
advertisement

Light as a particle: During the 1800’s the concept that light was a wave was the predominate theory, but in the early 1900’s a two experiments were done that could not be explained by that concept. These were: Black Body emissions by Maxwell Planck Hot objects give off light. Max Planck found that this light was not emitted continuously, but instead came off in discrete packets that Planck referred to as Quanta. Quantum – the minimum amount of energy that can be gained or lost by an atom Planck determined that the Energy was proportional to the frequency of the light. E ∝ f A proportional statement can be converted to an equality by multiplying by a constant. The constant that max Planck found was h = 6.626 x 10-34 Js. This is called Planck’s constant and results the equation, E = hf. Photoelectric Effect by Heinrich Rudolph Hertz When light shines on a piece of metal electrons are knocked free from the metal creating a current. (Slide 1) (Demo: Photovoltaic Cell with Voltmeter) Wave theory predicts that the electron flow would increase as the brightness increased. (Slides 2 and 3) However, experiments showed that brightness did not have an effect, but frequency did, the higher the frequency, the higher the current. (Slide 4) Each metal responds to a specific threshold frequency. Light at a lower frequency than this threshold will not cause and electron flow, no matter how bright the light. Electrons are only emitted by a metal when the metal is struck by a photon with the required energy. Duality Einstein explained the contradiction between the concepts of light as a wave vs particle by proposing that light is both a wave and a particle. Which one we find depends on the conditions of the experiment. The quantum determined by Planck is carried by a photon. Photon – particles of electromagnetic radiation with zero mass and 1 quantum of energy. Light is studied by looking at the spectrum created by passing the light through either a prism or a diffraction grating. The spectra produced fall into two categories; Continuous spectra and Emission (Line) Spectra. Continuous spectra – the colors blend from one to the other producing an unbroken sequence from red to violet. This is the spectrum that we see if we look at white light. Emission (Line ) Spectra – A series of distinct colored lines. Each color is a specific frequency, with a specific amount of energy. This is the type of spectra produced by passing electricity through a gaseous element, with each element having its own unique spectrum that can be used to identify it. The concept of Duality of light allows us to combine the wave and particle equations, since both include frequency. 𝐜 𝐄 𝐟= = 𝛌 𝐡 Problems: 1) Determine the amount of energy in a photon of gamma radiation with a frequency of 5.00 x 10 18 Hz. 𝐸 = ℎ𝑓 = (6.626 𝑥 10−34 𝐽𝑠) ( 2) 5.00 𝑥 1018 ) = 3.31 𝑥 10−15 𝐽 𝑠 A photon of for a radio wave has 1.988 x 10 -24 J, what is its frequency? 𝐸 1.988 𝑥 10−24 𝐽 𝑓= = = 3.000 𝑥 109 𝐻𝑧 ℎ 6.626 𝑥 10−34 𝐽𝑠 3) A quantum of infrared radiation has 3.439 x 10-21 J of energy. What is the wavelength of this radiation? (3.00 𝑥 108 𝑚/𝑠)(6.626 𝑥 10−34 𝐽𝑠) 𝑐ℎ 𝜆= = = 5.78 𝑥 10−5 𝑚 𝐸 3.44 𝑥 10−21 𝐽 4) How much energy is in a photon of Ultraviolet radiation with a wavelength of 9.87 x 10-7 m? (3.00 𝑥 108 𝑚/𝑠)(6.626 𝑥 10−34 𝐽𝑠) 𝑐ℎ 𝐸= = = 2.01 𝑥 10−19 𝐽 𝜆 9.87 𝑥 10−7 𝑚