Ethics Screening Guidelines and Checklist
advertisement
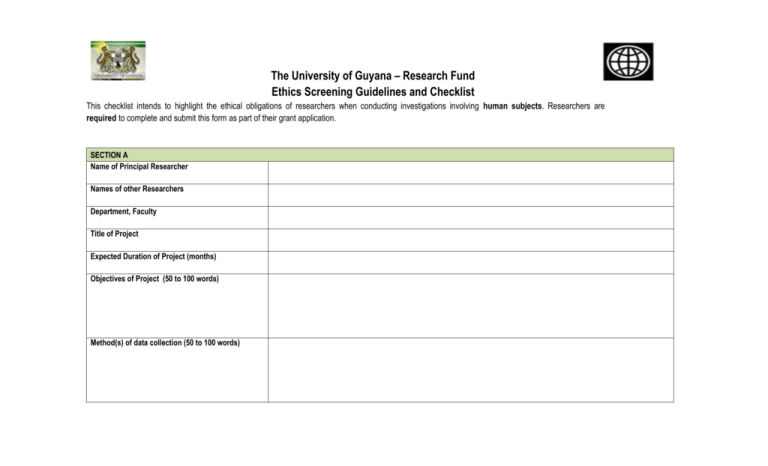
The University of Guyana – Research Fund Ethics Screening Guidelines and Checklist This checklist intends to highlight the ethical obligations of researchers when conducting investigations involving human subjects. Researchers are required to complete and submit this form as part of their grant application. SECTION A Name of Principal Researcher Names of other Researchers Department, Faculty Title of Project Expected Duration of Project (months) Objectives of Project (50 to 100 words) Method(s) of data collection (50 to 100 words) SECTION B Ser # Question Yes No Not Applic able Measures to address Ethical Issues 1 Does the study involve participants who are legally or otherwise not in a position to give their valid consent to participate (examples: children, prison inmates, mental patients)? If yes, use this space to provide details of the measures to be taken to ensure the protection of the human subjects and to adhere to the universal ethical principles of respect, informed consent, confidentiality, justice and minimization of harm. 2 Will the participants be informed of the nature of their involvement in the collection of data of all features of the research that reasonably might be expected to influence willingness to participate? If no, use this space to provide details of the measures to be taken to ensure the protection of the human subjects and to adhere to the universal ethical principles of respect, informed consent, confidentiality, justice and minimization of harm. 3 Will the participants be told that they can discontinue their participation at any time? If no, please use this space to provide strong evidence why this is necessary. Also a clear indication of what the possible harm to participants will be and what will be in place in the study to prevent and or mitigate this harm. 4 Will the participants in your study be aware that they are participants? If no, please use this space to provide strong evidence why this is necessary. Also a clear indication of what the possible harm to participants will be and what will be in place in the study to prevent and or mitigate this harm. 5 Will participants or their guardians be asked to sign Consent Forms? If not, will participants/guardians be giving verbal consent? If yes, please attach the instruments and/or scripts to be used in obtaining consent. 6 Will financial inducement (other than reasonable expenses and compensation for time) be offered to participants? If yes, please use this space to provide details. In addition, please attach the instruments and/or scripts to be used to obtain consent. 7 Is confidentiality of the participant's identity positively ensured? 8 Will this research expose participants to any significant risk of physical or emotional harm? 9 Could publication of the research results possibly interfere with strict confidentiality? Could publication of the results possibly harm the participants - either directly or through identification with his/her membership group? Are there other aspects of this study that may interfere with the protection of the well-being and dignity of the participants? 10 11 If yes. Please use this space to provide evidence of measures that would be undertaken to ensure confidentiality of the subjects identity and other personal information? If no, use this space to provide details of the measures to be taken to ensure the protection of the human subjects and to adhere to the universal ethical principles of respect, informed consent, confidentiality, justice and minimization of harm. If yes, please use this space to provide details of the measures to be taken to prevent and mitigate harm. If yes, the participants should be told upfront and given the option to withdraw from the study. If yes, what protections and mitigation measures will be in place. If yes, use this space to provide details and accompanying measures to be taken to ensure the protection of the human subjects and to adhere to the universal ethical principles of respect, informed consent, confidentiality, justice and minimization of harm. In addition to the above, researchers MUST: 1. Respect the right of the participants to withdraw his/her data in cases where there is a possibility that the participant's identity can be deduced by someone other than the researcher; 2. Take all necessary measures to protect the physical safety of the participants (from dangers such as faulty electrical equipment, poor grounding, lack of oxygen, falls, traffic and industrial accidents, the possibility of hearing or vision loss, and so forth); 3. Ensure that non-coded information obtained on individual participants is never disclosed to third parties; and 4. Undertake to ensure that findings of the research are shared with the participants and their community. By appending my signature, I hereby swear to undertake all measures included in this form to address ethical issues highlighted. Signed by Principal Researcher: __________________________ Date: __________________________ ETHICAL APPROVAL GIVEN [ ] ETHICAL APPROVAL WITHHELD PENDING REVISIONS BASED ON COMMENTS OF THE ERC [ ] ETHICAL APPROVAL WITHHELD DUE TO SERIOUS CONCERNS IN PROPOSALS [ ] Signed by Chairperson of Research Committee: _________________________ Date: _________________________










