(MSc) in Biopharmaceutical Science. - Online Learning
advertisement

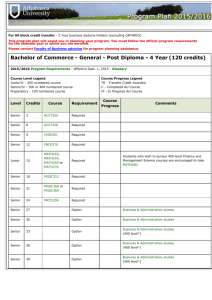
Postgraduate Diploma and Master of Science (MSc) in Biopharmaceutical Science. OVERVIEW. The joint IT Sligo/NIBRT Post-graduate Diploma and Masters in Biopharmaceutical Science aims to provide scientists and engineers who have industrial or equivalent experience with a comprehensive grounding in critical aspects of Biopharmaceutical Processing and Support Services, with specific focus on the product lifecycle of Biopharmaceutical products and processes. It has been designed to provide graduates with a range of cutting-edge skills in Biopharmaceutical Science and Medical Biotechnology thereby making them highly employable within the Biotechnology, Biopharmaceutical and Medical Biotechnology Industries in Ireland and abroad. Medical Technology and Biopharmaceutical companies are among the fastest growing in the Life Sciences sector in Irish industry at present. One of the key features of this programme is that it combines skills in Biopharmaceutical Science and Medical Biotechnology with an in-depth knowledge of legislation/regulation and quality control systems for the respective industries. COURSE STRUCTURE. The structure of the programme is a part-time two-year format comprising modules of either 5 or 10 credits each, totalling 60 credits, which leads to the award of Postgraduate Diploma in Biopharmaceutical Science (Level 9), granted by the Institute of Technology Sligo. A dissertation for a further 30 credits over an additional 1 year period will lead to the award of M.Sc. in Biopharmaceutical Science. The programme is delivered primarily through distancelearning / online learning technologies. The programme is designed to provide participants with a comprehensive grounding in critical aspects of Biopharmaceutical processing and corresponding support service areas. The programme is run in conjunction with NIBRT (National Institute for Bioprocessing Research and Training), which is the leading body in Ireland for the development of Biologics Processing expertise comprising both training and research. This programme will avail of the specialist training facilities provided by NIBRT including elective practical laboratory based modules and pilot plant scale operations. PROGRAMME CONTENT. The programme requires the completion of six core/mandatory modules, addressing the fundamental areas in Biopharmaceutical processing. A further six elective modules are available from a wide choice of modules across 3 principal strands as follows: Operations, Science, and Quality System/Compliance. Students take modules to the value of 60 Credits for the post-graduate diploma. The 6 core modules are as follows: Biopharmaceutical drug discovery and development Fermentation and cell culture processing Biopharmaceutical analytical techniques. Protein purification processing Legislation/Regulatory Affairs for Biopharmaceutical products Biocontamination monitoring and control Elective modules are chosen from the three principal strands. Quality System/Compliance Strand: Bioprocess scale-up and technology transfer Biopharmaceutical industry regulation and management Biopharmaceutical process validation Lean Sigma principles for biopharmaceutical processing Biopharmaceutical risk assessment and management Research Methods Science Strand: Formulation and delivery of biopharmaceuticals. Biocontamination Monitoring and Control. Advanced glycobiology analysis. Practical Based Modules: ([Elective modules] If chosen, will require short blocks of attendance at the NIBRT facility). Upstream Processing Practical Module Downstream Processing Practical Module Bipharmaceutical Analytical Techniques Practical Module Operations Strand: Biopharmaceutical facility design and operation Utilities for biopharmaceutical processes Project management for biopharmaceutical projects Upstream Bioprocessing Engineering Downstream Bioprocessing Engineering Bioprocess design and operations SKILLS SET ACHIEVED. DNA Technology and Genetic Engineering. Cell Culture Processing. Protein Purification. Bioanalytics.. Biopharmaceutical Validation. Regulatory Affairs, Legislation and Compliance. Bioprocessing Laboratory Practical Techniques. Formulation and Fill Finish for Biologics. Upstream and Downstream Engineering. Lean Sigma for Biologics Processing. Biocontamination Monitoring and Control. Bioprocess Design and Operations. ASSESSMENT AND DELIVERY. Currently this course is assessed and delivered 100% on-line but if the elective practical laboratory based modules are chosen they will require short blocks of attendance at IT Sligo or the NIBRT facility. Some or all of the following assessment modes may apply: examinations, practical work, projects (both individual and group), essays, assignments and on-line vivas. CAREER OPPORTUNITIES. Post-graduates from this programme typically find employment in Scientific, Operations and Quality Assurance/Control positions within the Biopharmaceutical and Biotechnology industry sectors both in Ireland and abroad. Their work may be involved in the manufacture of novel medicines from living cells, immunodiagnostics, bioanalytics, process validation and process optimisation. Medical Biotechnology and Biopharmaceutical Processing are among the fastest growing industry sectors in Ireland and internationally at present and with the development towards a knowledge-driven economy they are likely to remain at the forefront for the foreseeable future. Post-graduates may also find employment more broadly within the Healthcare sector and in areas of Research and Development. ENTRY REQUIREMENTS. 1. To obtain entrance to the Postgraduate Diploma (60 Credits): Candidates must have achieved a Level 8 Honours Degree in Science or Engineering awarded by a recognised Degree awarding body or Hold a relevant National Diploma/Ordinary degree in Science or Engineering and have obtained a minimum of three years relevant post qualification experience. Candidates obtaining honours in the Postgraduate Diploma may undertake the additional 30 credits by way of a dissertation for the M.Sc programme. 2. To obtain entrance to the M.Sc programme directly (90 Credits): Candidates must have achieved a Level 8 Honours Degree or its equivalent in an appropriate discipline (i.e. Engineering or Life Sciences). DURATION. Typically the duration of the programme will be part-time over 2 years for the postgraduate diploma and an additional 1 year for the dissertation. The course commences in September each year. Closing date for online applications is Sept. 7th 2012. Online Application link: https://ssb.ancheim.ie/its/app/bwskalog.P_DispLoginNon FURTHER INFORMATION Sarah Walsh, IT Sligo. Lifelong Learning Tel: 071 91 37249, walsh.sarah@itsligo.ie odl@itsligo.ie WEBSITE. http://odl.itsligo.ie/science/bio-pharmaceutical-courses/msc-in-biopharmaceutical-science/