D.R. 2.1 - Chemistry 9th ed

SVHS ADVANCED BIOLOGY NAME: ____________________
Tortora 9 th edition PERIOD: 1 2 3 4 5 6
D.R. #2.1 – CHEMISTRY
AFTER READING PAGES 25-31, ANSWER THE QUESTIONS BELOW.
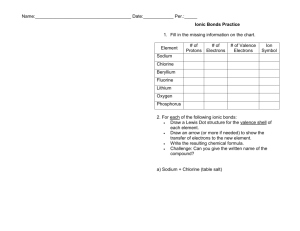
1. Using Table 2.1, discuss what percent of the body are Carbon, Hydrogen, Oxygen, and Nitrogen in the human body?
What are the major roles of these elements?
2. What information does the atomic number and atomic mass give you about an atom? Why is the atomic mass on the Periodic Table usually a fraction?
3. How are electron “clouds” different than shells? How many electrons can each of the first three shells hold?
4. In Fig 2.2, how many electrons are in the outer shell of H, C, O, and N?
5. What are valence electrons by definition? How do they play an important role regarding chemical bonding?
6. Using Fig 2.2, look at the valence electrons of the atoms shown. How many Hydrogen atoms would you expect to bond covalently with Oxygen?_____ Hydrogen with Carbon?____ Hydrogen with Nitrogen? ____
Oxygen with Carbon?________
7. Using Fig. 2.2, look at the Sodium and Chlorine atoms. How many electrons in the outer shell do each atom
have? ______ Why will these two types of atoms form an ionic bond and not a covalent bond?
8. Using Fig. 2.2, why does Chlorine want to gain 1 electron?
Explain what the “rule of eight” refers to using the chlorine atom as an example.
9. Using Fig 2.3, explain the difference between atoms and molecules.
Are atoms joined by ionic bonds considered molecules?
10. What is a free radical?
How can consuming antioxidants counteract reactions of free radicals?
11. Why are the electrons colored orange in Fig 2.5? Explain why O 2 has two bonds but shows 4 orange electrons?
12. Using Fig 2.5 explain why electrons in Hydrogen gas and Oxygen gas are shared equally :
13. Describe a polar covalent bond, and give a common example:
14. Explain why Hydrogen bonds form between water molecules: