0581.5271 – “Electrochemistry for Engineers” HOMEWORK #2
advertisement

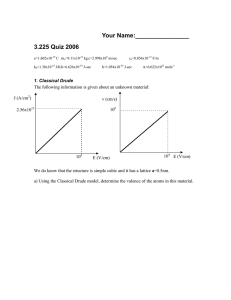
0581.5271 – “Electrochemistry for Engineers” HOMEWORK #2 Question 1) Consider the reduction of sodium ions (Na+) into a spherical mercury drop electrode to form an Na-Hg amalgam. a) In order to observe the mass-transport limiting current for sodium reduction, do we need to be close-to or far-from the standard reduction potential? Why? b) Assuming mass-transport control, set up the proper PDE, initial ,boundary and semi-infinite conditions required to model the sodium concentration as a function of distance from the drop for a Cottrell experiment. c) Which condition (initial, surface, semi-infinite) would change if the potential step was small? Write the expression for the new condition assuming a potential step of arbitrary size. d) For small droplets, rO(s/DNa+)1/2 << 1, the equations in part (c) give the following solution in Laplace space: i( s) * nFADNa C Na (1 2 )rO s where rO is the radius of the Hg drop Is the measured current using this solution a function of time? Why or why not? Question 2) Imagine the electrochemical reaction Fe(CN)6 -3 + e- Fe(CN)6 -4 is occurring on the surface of a platinum electrode. a) What conditions must be present in order to assume that the surface concentration of both species is the same as the bulk? b) What conditions must be present in order to assume that the surface concentrations of both species obey the Nernst equation? c) Calculate the equilibrium rate constant, ko for the reaction assuming that the electrolyte has 2mM of both species, the transfer coefficient is 0.5, and the exchange current density is 2 mA/cm2 (make sure to show all units!) d) Assuming the oxidized species was the only species initially present at a concentration of 1M, plot the current density we can expect from the electrode after a potential step from (E-Eeq) = +500 mV to (E-Eeq) = -1,800 mV. You may assume that Fe(CN)6 -3 has a diffusion coefficient of 6.6e-6 cm2/s