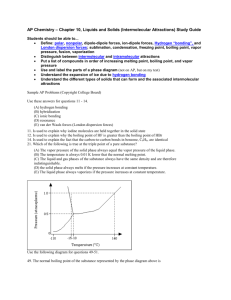
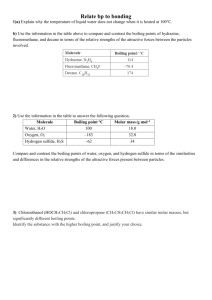
57 odd. 49. Determine the kinds of intermolecular forces that
advertisement

Chapter 11 -- 49 – 57 odd 49. Determine the kinds of intermolecular forces that are present in each of the following elements or compounds: a. Kr—London Dispersion d. HF—Hydrogen Bonding g. CO—London Dispersion b. 𝑁𝐶𝑙3-- Dipole-Dipole c. SiH4 – London Dispersion e. N2—London Dispersion f. NH3—Hydrogen Bonding h. CCl4 – London Dispersion 51. Arrange the following in order of increasing boiling point. Explain. a. CH4 b. CH3CH3 c. CH3CH2Cl d. CH3CH2OH A, B, C, D A has the lowest boiling point because it is nonpolar and has the lowest molar mass. B has the 2nd lowest boiling point because it too is non-polar but has a larger molar mass. C is the 3rd lowest boiling point because it is polar and displays dipole-dipole intermolecular forces. D has the largest boiling point because of its molar mass, it also contains hydrogen bonding which is the strongest type of intermolecular force, giving it the highest boiling point. 53. For each pair of compounds, pick the one with the highest boiling point. Explain your reasoning. a. CH3OH or CH3SH -- CH3OH has the highest boiling point because of H-bonding. b. CH3OCH or CH3CH2OH -- CH3CH2OH has the highest boiling point because of H-bonds. c. CH4 or CH3CH3 -- CH3CH3 has a higher molar mass so more London Forces are present. 55. For each pair of compounds, pick the one with the higher vapor pressure at a given temperature. Explain your reasoning. a. Br2 or I2 -- I2 has less of a dipole-dipole and a lower boiling point and a higher vapor pressure b. H2S or H2O -- H2S lacks hydrogen bonds, has a lower boiling point and high vapor pressure c. NH3 or PH3 -- PH3 No hydrogen bonding, lower bp, and a high vapor pressure. 57. Which of the following pairs of substances would you expect to form homogenous solutions when combined? For those that form homogeneous solutions, indicate the type of forces that are involved. a. CCl4 and H2O b. KCL and H2O c. Br2 and CCl4 d. CH3CH2OH and H2OLike dissolves Like – H-bonding. 59. Which compound would you expect to have greater surface tension, acetone [(CH 3)2CO] or Water (H2O)? Explain. Water has a higher surface tension due to the hydrogen bonding present.