Kelly - End of Summer Report.doc
advertisement

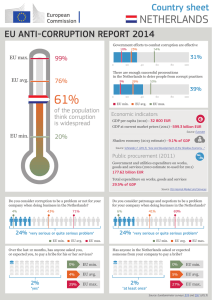
Teacher Name: School Campus / ISD Name: Classroom Subject: Grade Level: Time Frame: Linda Kelly John Marshall High School / Northside ISD / San Antonio, TX Chemistry pre-AP and Physics I 10-11 3 to 4 days during the first six weeks of school End-of-Summer Report Overview: My E3 laboratory assignment consisted of viewing ammonium perchlorate crystals (used in rocket propellant) under a light microscope and photographing the crystals. I used Photoshop, IQ Materials software, and Excel to gather data and calculate the particle size and the distribution of these sizes. From this work, I propose to have the chemistry and physics students perform a similar investigation. I believe this research is relevant to my students because they continually ask, “Are we going to blow something up?” Almost everyone in interested in rockets or have seen fireworks displays. Because of my lab experience, I can better explain some of the synthesis procedures in creating rocket propellant. Likewise, I can explain the importance of using exacting measurements, record keeping, and repeated trials (not just doing one trial as time constraints in a classroom setting permit). The students would complete a pre-Test of calculations and engineering questions. I would show a PowerPoint presentation explaining my actual lab research. During whole-class-instruction, I would discuss quantities, solve some metric conversions, demonstrate how to use the electronic balance, and how to read a meniscus with a graduated cylinder. The students would collect their data and make graphs in the next two class periods. Then I would end this lesson with a post-test. TAKS Science Objectives: 1 (Nature of Science) 4 (Chemistry) Math Objectives: 4 (equations & inequalities) 8 (measurement & geometry) 9 (Statistics) TEKS: Chemistry 1,2,3 (investigations & measurement) Physics 2 Math +,-,x,/ Technology: All groups graph the size distribution of particles in the sample using Excel spreadsheet and graphing capabilities; calculators Task: Students will determine the mathematical attributes and size distribution of a sample of “rocket propellant.” Given samples, students determine mass, volume, density, surface area, size distribution. Students will use appropriate units, use significant figures, write scientific notation, show sample calculations, graph surface area vs. distribution of particles. My classroom project consists of groups of students measuring and solving calculations independently and simultaneously. Students will engage in lecture, hands-on laboratory investigations, cooperative learning, problem solving, technology integration, and communicating results. As a class, we will determine which method gives the better results: direct or indirect measurement. Procedure: Set A: Half the students (8 lab groups of 2 students/group) will gather data from direct measurement. Students will measure diameters, mass, measure volume by water displacement, calculate radius, density, surface area of their sample; graph particle size distribution by counting and calculation. Set B: Half the students (8 lab groups of two’s) will gather data through indirect measurement via a photograph and centimeter ruler. Students will measure diameters, find radius, use the formula for “volume of a sphere” to calculate volume. Given the total mass of a sample and the percent of each size, students would perform percent by mass calculations to determine the mass of each size of particles. Students must collect data for a representative sample—one measurement is not enough. Challenge: Which method (actual counting vs. photography) will give the best results in a timely manor? I hope that the results from similar samples will correspond using these different methods. Given the lab equipment, I expect the students to develop their own procedures for collecting data. Lab equipment: electronic balances, beakers, graduated cylinders, water, rulers, computers Teacher set up: Glass Pearl Beads (Wal-Mart jewelry craft #36402 $8.96 / 285 g of beads) Plastic Petri dishes (60mm from biology lab or Analytical Scientific $13.99 / 20 ) Place beads (several small, medium, large) in dishes. Tape lids to bottoms. A sample may include beads of varying sizes, different colors (contaminants present), not filling the dish completely (deal with mobile particles), beads stuck in a matrix of slime or Elmer’s Glue (cannot remove beads out of environment), another foreign substance being mixed with the beads, etc. Assume all the small beads are the same; all the medium beads are the same, all the large beads are the same. Assume beads are perfect spheres. Take a digital photo of each of the samples. Reduce or enlarge the photo until a printed version measures bead size at 10, 8, 6 mm. The students will have to physically measure diameters of the particles and calculate volume and surface area of spheres. I do not know how they calculate density from a photo unless mass is given. However, given the total mass of the sample and percent distributions of the particles, students can calculate percent composition of each size. (43 % are large beads x 14.23 g total mass = 6.119 g attributed to all the large beads.) Correlations: 1. The sample is a chemical crystal. Calculate the actual size of an average crystal in micrometers ( μm ) using scientific notation. 2. The sample represents an image received from the Hubble telescope. Calculate the actual size of an average star in kilometers ( km ) using scientific notation. Evaluation: The investigation would be evaluated by the precision of several classes of students calculating the average size of the samples. The lab would be evaluated by the accuracy of the student answers and meeting the challenge of which method derives the best answers in a timely manor. Expected Outcomes: I expect the students will be reluctant to perform a lab without directions, but then to attempt measuring when they see others working. I expect the students to perform the objectives as stated in the TEKS. I expect better results from direct measurement because photographs of spheres may be distorted due to parallax. I expect students to appreciate the many measurements researchers must make in order to find a true meaning in their data. I expect the students to answer more post-test questions correctly. I expect the students to gain a better understanding of the work of a researcher, scientist, or engineer. I expect the students to have fun working together. Supplemental pages following: Direct Measurement Data page Indirect Measurement Data page Particle Size Lab Direct Measurement Data sample # _______ names______________________________ Measure the sizes of the particles in your sample. Then calculate the average for one particle. Be sure to specify units. Mass ____ Large = _____________ avg = _______________ ____ Med = _____________ avg = _______________ ____ Small = ______________ avg = _______________ ____ Total = ______________ Volume ____ Large = _____________ avg = _______________ ____ Med = _____________ avg = _______________ ____ Small = ______________ avg = _______________ ____ Total = ______________ Density ____ Large = _____________ avg = _______________ ____ Med = _____________ avg = _______________ ____ Small = ______________ avg = _______________ ____ Total = ______________ Surface Area The formula for surface area of a sphere is __________________ Given the volume, find r ____ Large = _____________ avg = _______________ ____ Med = _____________ avg = _______________ ____ Small = ______________ avg = _______________ ____ Total = ______________ Graph of distribution Surface Area vs. particle size Particle Size Lab Indirect Measurement Data photo # _______ names______________________________ Measure the sizes of the particles in your sample. Then calculate the average for one particle. Be sure to specify units. Mass decimal percent x total mass = mass of one particle ____ Large = _____________________ avg = _______________ ____ Med = _____________________ avg = _______________ ____ Small = ______________________ avg = _______________ ____ Total = ______________________ Volume The formula for the volume of a sphere is _____________________ ____ Large = _____________ avg = _______________ ____ Med = _____________ avg = _______________ ____ Small = ______________ avg = _______________ ____ Total = ______________ Density ____ Large = _____________ avg = _______________ ____ Med = _____________ avg = _______________ ____ Small = ______________ avg = _______________ ____ Total = ______________ Surface Area The formula for surface area of a sphere is __________________ ____ Large = _____________ avg = _______________ ____ Med = _____________ avg = _______________ ____ Small = ______________ avg = _______________ ____ Total = ______________ Graph of distribution of Surface Area vs. particle size