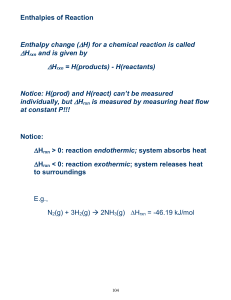
Enthalpy and Stoichiometry of DHrxn 71. Consider the following
advertisement

Enthalpy and Stoichiometry of Hrxn 71. Consider the following equation for the combustion of acetone (C3H6O), the main ingredient in nail polish remover. C3H6O + 4 O2 3 CO2 + 3 H2O Hrxn = -1790 kJ If a bottle of nail polish remover contains 155 g of acetone, how much heat would be released by its complete combustion? 72. The equation for the combustion of CH4 (the main component in natural gas) is shown below. How much heat is produce by the complete combustion of 237 g of CH4? CH4 + 2 O2 CO2 + 2 H2O Hrxn = -802.3 kJ 73. Octane (C8H18) is a component of gasoline that burns according to the following equation: C8H18 + 25 /2 O2 8 CO2 + 9 H2O Hrxn = -5074.1 kJ What is the mass of octane (in g) required to produce 1.55 x 103 kJ of heat? 74. The evaporation of water is endothermic as indicated by the following: H2O (l) H2O (g) Hrxn = +44.01 What minimum mass of water (in g) has to evaporate to absorb 175 kJ f heat?