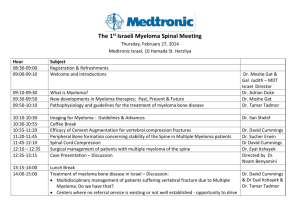
research - University of Washington
advertisement

Treatment Decision Making in Older Adults Newly Diagnosed with Myeloma Joseph D. Tariman A dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy University of Washington 2011 Program Authorized to Offer Degree: School of Nursing University of Washington Graduate School This is to certify that I have examined this copy of a doctoral dissertation by Joseph D. Tariman and have found that it is complete and satisfactory in all respects, and that any and all revisions required by the final examining committee have been made. Chair of the Supervisory Committee: ___________________________________________________ Donna L. Berry Reading Committee: __________________________________________________ Donna L. Berry __________________________________________________ Barbara B. Cochrane __________________________________________________ Ardith Doorenbos ___________________________________________________ Karen G. Schepp Date: _________________________________ In presenting this dissertation in partial fulfillment of the requirements for the doctoral degree at the University of Washington, I agree that the Library shall make its copies freely available for inspection. I further agree that extensive copying of the dissertation is allowable only for scholarly purposes, consistent with “fair use” as prescribed in the U.S. Copyright Law. Requests for copying or reproduction of this dissertation may be referred to ProQuest Information and Learning, 300 North Zeeb Road, Ann Arbor, MI 48106-1346, 1-800-521-0600, to whom the author has granted “the right to reproduce and sell (a) copies of the manuscript in microform and/or (b) printed copies of the manuscript made from microform.” Signature ________________________ Date ____________________________ University of Washington Abstract Treatment Decision Making in Older Adults Newly Diagnosed with Myeloma Joseph D. Tariman Chair of the Supervisory Committee: Affiliate Professor Donna L. Berry Department of Biobehavioral Nursing and Health Systems Myeloma is a plasma cell malignancy of the elderly, affecting over 20,000 individuals annually. The advent of novel agents has increased the number of treatment options for older adults newly diagnosed with myeloma, but it has also led to clinical uncertainties. These uncertainties regarding optimal therapy are due in part to the lack of randomized controlled trials comparing new treatment options over the existing options. There is considerable evidence that patients want to be informed and consulted with regard to the impact of treatment, not only on survival but also on quality of life. However, no data exist regarding the process of treatment decision making and degree of patients’ control preferences over treatment decisions in older adults diagnosed with myeloma. This dissertation study employed a multi-method, cross-sectional survey design. Physicians and older adults diagnosed with myeloma, ages 60 years and above were interviewed separately to describe how treatment decisions were made from both perspectives. After the interview, each participant was asked to report their degree of preferred control over treatment decisions using the Control Preference Scale (CPS) and to complete the Information Needs Questionnaire to assess their information priorities. The results revealed that patient and physician participants have various treatment considerations to arrive at a possible “best treatment decision.” A high percentage of these older adults with myeloma want some control of the treatment decision (45% preferred shared and 50% active roles). Their top three priority information needs related to “different types of treatments” and corresponding advantages/disadvantages, the “likelihood of cure” and “caring for myself at home.” No statistically significant differences in decisional role preferences and priorities of information needs were seen among various sociodemographic factors. Since older adults newly diagnosed with myeloma will most likely prefer to participate in treatment decision-making, physicians should assess their preferences. Physicians should elicit from patients their treatment preferences to account for contextual factors that might be overlooked if their opinions on therapies are not solicited. Health care professionals should assess and address the priority of information needs of myeloma patients and encourage patients to express their decisional role preferences to their physician. TABLE OF CONTENTS Page List of Figures ..................................................................................................................... iii List of Tables ....................................................................................................................... iv Acknowledgement .................................................................................................................v Dedication ............................................................................................................................ vi Chapter I: Problem Statement and Significance of the Study Introduction ................................................................................................... 1 Specific Aims of the Study ........................................................................... 3 References ..................................................................................................... 5 Chapter II: Literature Review of Treatment Decision Making in Cancer Introduction ....................................................................................................7 Physician Factors Influencing Treatment Decisions in Cancer .....................8 Patient Factors Influencing Treatment Decisions in Cancer ........................ 16 Theoretical Frameworks in Treatment Decision-Making ............................ 23 Decisional Role Preferences ........................................................................25 Priority of Information Needs in Cancer Patients ........................................28 Conclusion ...................................................................................................39 References ....................................................................................................41 Chapter III: Patient and Physician Perspectives on Treatment Decision Making in Older Adults Newly Diagnosed with Myeloma Introduction ..................................................................................................55 Treatment Considerations in Older Adults ..................................................57 Purpose .........................................................................................................59 Methods ........................................................................................................59 Results ..........................................................................................................63 Discussion ....................................................................................................84 Conclusion ...................................................................................................91 References ....................................................................................................92 i Page Chapter IV: Decisional Role Preferences in Older Adults Newly Diagnosed with Myeloma Introduction ................................................................................................. 98 Theoretical Framework ................................................................................99 Purpose .......................................................................................................101 Methods ......................................................................................................101 Results ........................................................................................................105 Discussion ..................................................................................................117 Conclusion .................................................................................................120 References ..................................................................................................122 Chapter V: The Priority of Information Needs in Older Adult Patients Newly Diagnosed with Myeloma Introduction ................................................................................................130 Purpose .......................................................................................................131 Methods ......................................................................................................132 Results ........................................................................................................142 Discussion ..................................................................................................151 Conclusion ................................................................................................ 155 References ..................................................................................................156 Appendix A: The Control Preferences Cards ....................................................................161 ii LIST OF FIGURES Page Chapter IV: Figure Number 1. Distribution of Respondents On and Off ABCDE Metric ............................................ 108 Chapter V: Figure Number 1. Priority of Information Needs by Rank .........................................................................151 iii LIST OF TABLES Page Chapter II: Table Number 1. Degner and Beaton’s Pattern of Control ......................................................................... 25 2. Summary of Information Needs Priorities .......................................................................30 Chapter III: Table Number 1. Sociodemographic Characteristics of Patient Sample .....................................................64 2. Sociodemographic Characteristics of the Physician Sample ...........................................65 3. Major Themes from Patient Participant Interviews ......................................................... 65 4. Major Themes from Physician Participant Interviews .....................................................68 5. Patient-specific Factors Influencing Treatment Decisions ..............................................72 6. Contextual Factors Influencing Treatment Decisions ...................................................... 74 7. Physician-specific Factors Influencing Treatment Decisions ..........................................78 Chapter IV: Table Number 1. Degner and Beaton’s Patterns of Decision Making ...................................................... 100 2. Sociodemographic Characteristics of the Sample .......................................................... 106 3. Rank Ordering of Competing Scale Models ..................................................................107 4. Distribution of Decisional Role Preferences ..................................................................109 5. Decisional Role Preferences by Age ..............................................................................110 6. Decisional Role Preferences by Education ....................................................................110 7. Decisional Role Preferences by Income ........................................................................110 8. Patient’s Decisional Role Preference, Category, and Description .................................111 Chapter V: Table Number 1. Nine Information Items in INQ ………………………………………………………. 134 2. Gulliksen & Tukey and Mosteller’s Chi-square for INQ-Myeloma …………………. 138 3. Circular Triads ...............................................................................................................140 4. Kendall’s Coefficient of Agreement ………………………………………………….. 141 5. Sociodemographic Characteristics of the Sample .......................................................... 142 6. Frequencies of Participants’ Preference for 36 Paired Items in the INQ ....................... 144 iv ACKNOWLEDGEMENTS This work would not have been possible without the excellent mentorship of Donna L. Berry (Supervisory Committee Chair) and Barbara B. Cochrane (co-sponsor of my NRSA grant), and the support of my dissertation committee members Ardith Doorenbos and Karen G. Schepp. Special thanks to Helene Starks for serving as my Graduate School Representative. My sincere appreciation also goes out to Jerald R. Herting, Kevin Cain, and Jeff Sloan for their statistical support. I would also like to acknowledge the recruitment assistance provided by Aimee Kohn, Lynley Fow, Pamela Becker, Elaine Zedella, Judy Petersen, Kenda Burg, Seema Singhal, Jose Velasquez, and Tom Blackney. I also wish to thank the UW School of Nursing Department of Biobehavioral Nursing and Health Systems for the research training and seminars they provided me throughout my doctoral studies. This work was generously supported by the National Institutes of HealthNational Institute of Nursing Biobehavioral Training Grant (grant number T32 NR07106 ) and a National Research Service Award Grant (grant number 1 F31 NR011124-01A1), the Achievement Rewards for College Scientists (ARCS) Fellowship 2006-2009, Seattle chapter, and the Hester McLaw’s Nursing Scholarship. v DEDICATION This work is dedicated to my partner J. Todd Babcock, who has been a loving companion throughout the many years of my scholastic journey. vi 1 CHAPTER I Problem Statement and Significance of the Study Introduction Multiple myeloma is a disease of the elderly, affecting over 20,000 individuals annually with a median age at the time of diagnosis of 70 years.1 The advent of novel agents like bortezomib and lenalidomide has increased the number of treatment options for older patients newly diagnosed with myeloma, but it has also led to clinical uncertainties as to which of the new therapies might be optimal for a particular patients. These uncertainties are due in part to the lack of randomized controlled trials comparing the new treatment options with the existing options. Additionally, older adults have been under-represented in clinical trials, which has led to a paucity of evidence-based treatment guidelines in this patient population. All of these factors complicate the decision-making process, supporting the need for an in-depth examination of various influences impacting treatment decisions. Research on treatment decision making in the cancer patient population has focused primarily on the examination of patients’ decisional role preferences.2, 3 Both qualitative and quantitative findings support the conclusion that older patients want to be informed and consulted with regard to the impact of various cancer treatments, not only on survival, but also quality of life.4, 5 With other cancer diagnoses, in which adults have multiple choices, there is strong evidence that personal factors and the individual preferences of patients play an major role in how the treatment decision is made.6-11 However, we have very limited knowledge and understanding of the personal factors that older adults newly diagnosed with 2 multiple myeloma consider when making their treatment decisions. Additionally, we have limited knowledge of the relevant factors pertaining to treatment decisions that physicians consider, particularly their valuation of patients’ quality of life during the treatment decisionmaking process. A better understanding of control preferences over treatment decisions in older adults newly diagnosed with myeloma could raise physicians and other health care professional’s sensitivity to patients’ needs. Consequently, addressing patients’ decisional role preferences could lead to better patient participation and increased satisfaction with care. Improving decision satisfaction and reducing decisional conflict and decision-related anxiety and depression are important goals in cancer care.12 However, no data exist regarding how degree-of-participation influences treatment decisions or various personal factors ultimately impact decision outcomes in older myeloma patients. Assessing the priority of information needs in patients with cancer—and then providing the information needed—are both important tasks for physicians, advanced practice nurses, and nurses.13 Research has shown that providing the right kind of information to cancer patients leads to improved satisfaction with decisions, better coping with the stress of diagnosis, decreased psychological distress, and increased patient participation during treatment decision-making.14 In this era of scarce health care resources, providing the right information at the right time is paramount. Nurse interaction with cancer patients, while essential, is costly, and needs to be more efficient.15 Nurses spend more time with cancer patients than other health care professionals and are able to assess a patient’s information needs to prevent a communication breakdown. Older adults newly diagnosed 3 with myeloma need accurate, timely, and appropriate information to help them make autonomous decisions. The examination of both patient- and physician-related factors influencing treatment decisions and the assessment of patients’ decisional role preferences may help clinicians better understand the decision-making process. Understanding the important factors that influence a patient’s treatment decision and their decisional role preferences may help clinicians develop innovative ways to improve a patient’s satisfaction with their treatment decision. Finally, understanding a patient’s priority information needs can help guide clinicians in providing valuable patient education. Study Aims The overall purpose of this study was to examine treatment decision making in older adults newly diagnosed with myeloma. This study was conducted with the following specific aims: 1) Describe physicians’ perspectives on the decision-making process and their patients’ preferences for participation in decision-making. 2) Examine patients’ perspectives on the decision-making process including personal values, preferences for participation, and past experiences relevant to treatment decision-making. 3) Describe information needs and preferences for participation in decision-making of patients newly diagnosed with multiple myeloma. 4) Explore the association of sociodemographic variables with information needs priorities and preferences for participation in decision-making. 4 The remainder of this dissertation is formatted as four chapters, each comprised of a stand-alone paper. Chapter II is a review of literature and previous research findings relevant to this study, including a detailed discussion of the relevant physician- and patient-specific factors, including personal beliefs and values, preferences for participation, contextual factors, and health-related experiences influencing treatment choices. In Chapter III, the author describes the evaluation of physicians’ personal perspectives on the decision-making process and their perceptions of patient preferences for participation in decision making. The information needs priorities and preferences for participation in decision making in older adults newly diagnosed with myeloma, along with the association of sociodemographic variables with information needs priorities and preferences for participation in decision making are reported in Chapters IV and V. Implications for practice and future research are presented for each set of findings. 5 References 1. Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin. 2010;60(5):277-300. 2. Tariman JD, Berry DL, Cochrane B, et al. Preferred and actual participation roles during health care decision making in persons with cancer: a systematic review. Ann Oncol. 2010;21(6):1145-1151. 3. Singh JA, Sloan JA, Atherton PJ, et al. Preferred roles in treatment decision making among patients with cancer: a pooled analysis of studies using the Control Preferences Scale. Am J Manag Care. 2011;16(9):688-696. 4. Gaston CM, Mitchell G. Information giving and decision-making in patients with advanced cancer: a systematic review. Soc Sci Med. 2005;61(10):2252-2264. 5. Chouliara Z, Kearney N, Stott D, et al. Perceptions of older people with cancer of information, decision making and treatment: a systematic review of selected literature. Ann Oncol. 2004;15(11):1596-1602. 6. Berry DL, Ellis WJ, Woods NF, et al. Treatment decision-making by men with localized prostate cancer: the influence of personal factors. Urol Oncol. 2003;21(2):93-100. 7. Zeliadt SB, Moinpour CM, Blough DK, et al. Preliminary treatment considerations among men with newly diagnosed prostate cancer. Am J Manag Care. 2010;16(5):e121-130. 8. Knight SJ, Latini DM. Sexual side effects and prostate cancer treatment decisions: patient information needs and preferences. Cancer J. 2009;15(1):41-44. 6 9. Hawley ST, Lantz PM, Janz NK, et al. Factors associated with patient involvement in surgical treatment decision making for breast cancer. Patient Educ Couns. 2007;65(3):387-395. 10. Denberg TD, Melhado TV, Steiner JF. Patient treatment preferences in localized prostate carcinoma: the influence of emotion, misconception, and anecdote. Cancer. 2006;107(3):620-630. 11. Lee CN, Hultman CS, Sepucha K. What are patients' goals and concerns about breast reconstruction after mastectomy? Ann Plast Surg. 2010;64(5):567-569. 12. Weinfurt KP. Outcomes research related to patient decision making in oncology. Clin Ther. 2003;25(2):671-683. 13. Rutten LJ, Arora NK, Bakos AD, et al. Information needs and sources of information among cancer patients: a systematic review of research (1980-2003). Patient Educ Couns. 2005;57(3):250-261. 14. Husson O, Mols F, van de Poll-Franse LV. The relation between information provision and health-related quality of life, anxiety and depression among cancer survivors: a systematic review. Ann Oncol. 2010. Epub ahead of print. 15. Davison BJ, Goldenberg SL, Wiens KP, et al. Comparing a generic and individualized information decision support intervention for men newly diagnosed with localized prostate cancer. Cancer Nurs. 2007;30(5):E7-E15. 7 CHAPTER II A Literature Review of Treatment Decision Making in Cancer Introduction Treatment decision making is an important component of cancer care continuum. In older adults newly diagnosed with myeloma, various treatment options have many tradeoffs in terms of risks and benefits, ultimately impacting the patient’s quality of life. Nursing research on cancer treatment decision making (TDM) has been increasing over the past three decades. Several decision-making studies have focused on answering questions related to patients’ preferences for participation and information needs,1-6 which subsequently led to the development of intervention studies aimed at improving patient education and participation.7,8 A systematic review on information-giving and patient participation in decision making in patients with advanced cancer revealed that patients have unmet information needs and widespread misunderstanding of the disease, including its prognosis and treatment.1 In terms of preferences for participation, there is a huge variation in preferences, but more than half of patients want some control of the decision (collaborative and active roles).1, 3, 9 There is also discordance between patients’ preferred and actual level of participation, with some patients wanting more participation than what was actualized.3, 9 Overall, decision-making studies’ goals are directed toward achieving better patient outcomes, which include better compliance with treatment, increased patient satisfaction, reduced decisional conflicts, and decreased stress and anxiety from TDM.10 In the past, decision research has focused mainly on the physician, which was thought to be related to the dominant paternalistic model of the patient-physician relationship (where the physician makes the decision for the patient), commonly seen in practice from the 1960s 8 through 1980s.11-13 However, a shift in the physician-patient relationship paradigm from paternalistic to shared,14, 15 or more patient-controlled treatment decisions, is becoming more common in breast16, 17 and prostate18, 19 cancer patient populations. Consequently, decision researchers have also directed their focus more toward the examination of patient perspectives on medical decision making.17, 20, 21 For example, Berry and colleagues have explored the physician perceptions of personal and medical factors22 and the relevant aspects of treatment decision making reported by men with localized prostate cancer (LPC).23 Both patient and physician factors related to adjuvant chemotherapy use have also been examined in older women with breast cancer (≥65 years of age).24 This review highlights the relevant physician and patient factors that could influence treatment decisions in cancer patients. It also includes a brief review of the different models or frameworks of decision making and the decisional role preferences and priority of information needs among cancer patients. Moreover, this review examines the association of sociodemographic factors with patient’s decisional role participation and priorities of information needs. Finally, the current trend in patient participation during decision making is examined and implications for practice and research are suggested. Physician-related Factors Influencing Decisions in Cancer Treatment The physician-related factors influencing cancer treatment decisions include the physicians’ beliefs, attitudes, and values, medical expertise and practice type, medical and clinical factors, communication style, and the medical literature. Beliefs, Attitudes, and Values Physicians’ stated or implied beliefs, attitudes and values are relevant to cancer treatment decision making. Despite the fact that health-related quality of life (HRQOL) has 9 been reported as an important aspect of cancer treatment outcomes, particularly in older adults,25 there is evidence that clinical research trials in myeloma have not incorporated HRQOL as a study outcome.26 The authors of the 13 of 15 RCTs included in that review stated that the given HRQOL result should influence clinical decision making. However, HRQOL studies have had limited impact on the published guidelines for developing supportive care and treatment plans for patients with myeloma, probably because of weaknesses in study design and measures, plus differing interpretations of the results.26 Perhaps there is also a lower level of enthusiasm among investigators for HRQOL outcomes in myeloma clinical trials. Bouchardy and colleagues27 conducted a study which reviewed treatment outcomes in older women with breast and ovarian cancers and reported that the physician’s belief about who maintains control of the decision could play a role in the undertreatment of cancer. Undertreated women were found to have a significantly poorer prognosis, yet none of the reviewed studies examined factors that could lead to undertreatment. The authors did cite some potential reasons for undertreatment, which included the physician misconceptions and beliefs about diminishing benefits of treatment and beliefs that more harm than good will come from treatment. A physician’s belief that he or she should dominate the TDM process, plus an intentional disregard of full disclosure, were also suggested as among the potential reasons. In an ethnographic study involving 25 women with breast cancer, Freedman28 reported that the physician’s power to choose what is told to the patient and what is withheld is a powerful determinant in the medical decision-making process. Although this finding was supported in the report by only two illustrative cases, it underscores the possibility that when 10 a physician believes the patient is simply a passive recipient of care, the physician’s preferred treatment could trump the patient’s preference and influence treatment decisions. For example, in a study of 21 women with ovarian cancer, ages 47-77 (mean age, 60.6 years), the majority of participants (66%) perceived that the physician largely directed the interaction during the medical encounter and there were really no treatment choices offered and that they were simply told to have the therapy that the physician prescribed.29 Men with newly diagnosed LPC have also reported that the decision-making process was provider-led and they were passive recipients of decisions made by others. Cohen and Britten30 interviewed 19 men with LPC between the ages of 58 and 88 (mean age 74.4) using a semi-structured interview and found that patients perceived their treatment plans were mostly decided by their clinicians. These reports are surprising since they are contrary to a more recent study, which showed that 91.7% (N=133) of 145 men with newly diagnosed LPC were able to actualize their preferred decisional role participation.19 Perhaps this can be partially explained by the growing health care consumerism in North America. Some oncologists place a higher value on improvements in a patient’s overall survival, as compared to their patients, who place a higher value on quality of life (QOL). A study on decision making and QOL in older patients ages 60-85 years with acute myeloid leukemia or advanced myelodysplastic syndrome revealed that the physician’s opinion was influential in older adults’ decisions to undergo intensive chemotherapy. This study showed a physician preference for intensive chemotherapy, since this treatment is typically associated with improved overall survival rates. Unfortunately, as the chemotherapy treatment gets more intensive, it poses significant morbidity risks, which in turn affect the patient’s overall 11 QOL. Ninety seven percent (N=42) of 43 participants agreed with the statement that quality of life was more important to them than quantity of life.31 Ravdin, Siminoff and Harvey32 surveyed members of the National Alliance of Breast Cancer Organizations (NABCO), and 562 individual members responded. When asked what lowest degree of absolute benefit they found acceptable for a treatment option, the respondents’ median acceptable extension of life expectancy was 3 to 6 months, and the lowest acceptable reduction in recurrence risk was 0.5 to 1.0%. The researchers also noted that there was a considerable variation in outcomes expectations, with 27% of participants not considering anything less than 1 year of life expectancy as acceptable and 26% not accepting less than 5% reduction in recurrence risk as a worthwhile trade-off for the reduced HRQOL that inevitably accompanies treatment. Many women could not recall hearing quantitative survival information. Since treatment has an impact on patient’s QOL, the researchers suggested that physicians should give patients quantitative estimates of overall survival instead of qualitative descriptions in order to fully inform patients about survival benefits of the therapy.32 Collaboration between oncologists and primary care physicians (PCP) is important in the context of complex medical conditions, especially those seen in older adults with multiple comorbidities and advanced cancer. A collaborative relationship between oncologists and PCPs is vital since a patient’s PCP could be the patient’s primary source of health information relating to treatment decisions. A recent study33 showed that oncologists have various perspectives on how involved PCPs should be in terms of treatment and procedurerelated decisions in older adults with cancer. Fourteen percent of the oncologists surveyed believed that PCPs should be more involved with decisions. Additionally, the study showed 12 variability in oncologists’ reports on the frequency of their communication with PCPs about treatment goals or TDM. This variability indicates that oncologists may have very different preferences for PCPs’ participation in treatment or procedure-related decision making or too busy to communicate with PCPs, a factor that could potentially influence the decision and ultimately affect the patient’s satisfaction with that decision.33 Medical Expertise and Specialty Type The physician’s medical expertise and specialty type have been shown to influence treatment decisions as well. A recent survey of American Society of Clinical Oncology (ASCO) members on the management of sentinel node breast micrometastases (SNMM) revealed that radiation oncologists (76%) were more likely than medical oncologists (57%) or surgeons (47%) to consider axillary radiation instead of axillary dissection for SNMM (P = 0.0021). These findings are inconsistent with ASCO’s guidelines, which recommend axillary dissection as the primary treatment for SNMM in patients diagnosed with breast cancer.34 In another survey of physicians,35 Hodgkin’s lymphoma specialists were more likely to tailor therapy according to individual patient factors, while decisions of non-specialist physicians were influenced more by the number of Hodgkin’s lymphoma cases they have had in their practice. These findings were based on survey responses from 81 Hodgkin’s specialists and 73 randomly selected physicians from the American Society for Radiation Oncology (ASTRO) and ASCO membership lists. Moreover, physicians working in teaching institutions were more likely to choose combined modality therapy (CMT) over radiation therapy or chemotherapy alone.35 Although the overall survey response rate was only 50% (58% among Hodgkin’s lymphoma specialists and 43% for randomly selected oncologists), it 13 is important to note that 92% of the Hodgkin’s lymphoma specialists who responded were in teaching institutions, settings that typically follow established treatment guidelines such as CMT for Hodgkin’s disease. However, this study must be interpreted with caution, since there were limited treatment choices provided to the respondents and individual contextual factors were not accounted for during analysis. In patients with LPCs, a national survey documented that urologists tended to favor surgery, while radiation oncologists tended to favor radiation therapy over surgery.36 In an international survey, gastroenterologists favored surgery for the management of gastric lymphoma, while hematologists and oncologists were inclined to favor more conservative therapies.37 Overall, these studies support the notion that the physician’s expertise and type of practice influence treatment choices. Effects of a Lowered Life Expectancy with Increased Age on Treatment Offered Life expectancy and quality of life are two major factors in TDM, especially in patients diagnosed with cancer.38 The physician’s perception of a shorter-than-normal life expectancy has led to decreased adjuvant chemotherapy use among older adults diagnosed with stage III colon, breast, and non-small-cell lung cancers.39, 40 In two retrospective cohort studies using the Surveillance, Epidemiology, and End Results/Medicare-linked database, researchers found that physicians used implicit judgments about age and lowered life expectancy to decide whether or not they would utilize adjuvant chemotherapy after surgery for stage III colon cancer39 and breast cancer.41 Schrag et al.39 reviewed the records of 6,262 Medicare beneficiaries diagnosed with stage III colon cancer from 1991 through 1996. Study participants included mostly Caucasians ages 65-90 (mean age not reported), 24% of whom were at the bottom quartile of the median household income for their census track of 14 residence. The researchers found that the use of adjuvant chemotherapy after surgery declined dramatically with increasing chronologic patient age, after adjustment for potential confounders such as comorbidities. Among 3,391 patients with no comorbidities, the use of adjuvant chemotherapy was given in 80% of patients ages 65-69 years, 64% of whom were 75-79 years and 13% 85-89 years. These findings are consistent with a prospective study which revealed that a smaller proportion of patients with colorectal cancer above the age of 75 received surgery with chemotherapy as compared to those younger than 75.42 The researchers in that study suggested that the older patients should have received adjuvant therapy, since patients in their 70s and 80s continue to have a reasonably long life expectancy and arguably with good QOL. Overall, the study demonstrated that physicians’ assumptions of a lower life expectancy based exclusively on age may explain this low utilization of adjuvant chemotherapy among the elderly with colon cancer. A retrospective study examining the factors that influenced treatment decisions in older breast cancer patients at a single center found similar underutilization of treatments in this patient population. Hurria et al.41 reviewed records of 216 Memorial Sloan-Kettering Cancer Center (MSKCC) patients stratified into two age groups: 75-79 years and ≥80 years. These researchers found treatment differences in women with breast cancer ages 75-79 as compared to patients ages 80 and above. Patients in the older age group were less likely to receive an axillary lymph node dissection and radiation therapy. There were no data included in the report on non-medical factors that might influence a patient’s preference for not receiving therapy. The study revealed that the physician’s assumption of a lowered life expectancy in older adults impacted treatment decisions. Medical and Clinical Factors 15 Tumor type, cytogenetic profile, age-related physiologic decline, and the presence of other comorbidities influence physicians’ treatment decisions.21, 43 For example, chemotherapy is of greatest value in older adults with node-positive, estrogen receptornegative and progesterone receptor-negative breast cancer.40, 44 Chemotherapy decisions for older cancer patients generally involve adjustment of the dose based on the patient’s renal function, prophylactic use of growth factors, maintenance of hemoglobin levels around 12 g/dL and proper drug selection based on age-related pharmacokinetics.45 Communication Style Physicians’ communication patterns could inhibit older patients’ communication about their own treatment preference, potentially causing post-decisional dissatisfaction. A recent study46 of older patients with early-stage breast cancer showed that oncologists were significantly more verbal and direct with older adult patients than with middle-aged patients, in terms of expressing their treatment preference. According to the researchers, oncologists in the study sample appeared to change their communication with older patients to engage in smoother, slower, and more directive manner. The researchers suggested that these actions may be traced to traditional age stereotyping and recommended that oncologists should not be overly directive in their style of communication, but instead allow for patient participation during treatment consultations. Medical Literature Aside from comorbid conditions that are included as medical factors influencing treatment decision, physicians have also ranked the medical literature as an important factor they consider.21 In contrast, patients ranked social issues such as family preference and 16 family burden during therapy as more important. When physicians make evidence-based decisions, they are using the medical literature to support their treatment decisions.47 Patient-related Factors Influencing Decisions in Cancer Treatment The patient-related factors influencing cancer treatment decisions include the patients’ beliefs and values, ethnicity, decisional role preferences, health-related experience, perception of the decision making process, personal, and contextual factors. Beliefs and Values The patient’s personal beliefs and values have been shown to influence treatment decision-making. One study prioritized patients’ rankings of physician’s opinion, family preference, and family burden as important, respectively.21 These findings were corroborated by another study, in which older adults made their treatment decisions depending on the burden of the treatment, possible outcomes, and likelihood of adverse functional and cognitive outcomes.48 Using a structured questionnaire, Fried et al.48 surveyed the treatment preferences of 200 individuals who were 60 years of age or older (mean age 72.8) and had a limited life expectancy due to cancer, congestive heart failure, or chronic obstructive disease. Of these 200 patients, 79 had cancer (mean age 71.7) and were interviewed at home about treatment preferences according to three components of therapy: the burden it imposed, the possible outcomes, and the relative likelihood of each of these outcomes. The researchers found that when the possible outcome was survival with severe functional or cognitive impairment, respondents no longer wanted the therapy. This study illustrated that older patients’ treatment preferences may change in response to the perceived burden of therapy, quality of outcomes, and probability of achieving the desired outcomes. 17 In patients diagnosed with prostate cancer, a recent review has found that they put more value on survival than sexual function; however, in terms of the range of possible side effects from treatment, patients put more value on sexual function than other side effects.49 According to the authors of that review, men with newly diagnosed prostate cancer may have overestimated the risk of sexual problems associated with treatment for prostate cancer and have unmet information needs related to prostate cancer treatments. One could infer that the value patients place on certain HRQOL components may change depending on their understanding of available information. Quality of life has always been an important value for older adults, who have ranked it consistently at the top of their priorities.50-55 For example, among older adults, median age 72 years, with acute myeloid leukemia or advanced myelodysplastic syndrome, 97% (N=42) of 43 participants agreed QOL was more important than length of life, regardless of their therapy choice.31 Older adults (76-91 years old, mean age 83.4) with breast cancer (N=21) at various stages also ranked independence as a high-priority value.54 Ethnicity Although ethnic or national stereotypes are subjective and not universal, there are some documented commonalities among groups that are informative when considering factors influencing cancer treatment decisions. In a recent study of 329 Greek women diagnosed with breast cancer, researchers found that a great majority of patients (71.1%) preferred a passive role in decision making., This finding is in contrast to a lower proportion of passive preferences reported in similar breast cancer studies from North American and Western European countries.56 Another study reported that Korean Americans and Mexican Americans were more likely that non-Hispanic Caucasians to believe that the family should 18 make decisions about the use of life support.57 These first two groups are therefore more likely have a family-centered approach to decision making.57 According to this study, Caucasians valued individualistic beliefs (self-reliance, self-responsibility, and control) and were thus likely to hold to a “shared” model of decision-making57 while Hispanic and African American participants were more likely to value collectivism and to hold to a familycentered or paternalistic approach to decision making.58 These findings must be interpreted with caution because they are based on small studies with participants from specific geographical locations (in one case urban Southern California),57 which may differ significantly from other locations in both ethnic distribution and attitudes. A more recent review paper also concluded that Asians, particularly Koreans, preferred to share their decision making on end-of-life care with their families.59 Decisional Control (Role) Preferences Older adults often express a desire for shared decision-making, but the variation in their desire to participate in the process is substantial.1, 60-62 One recent study of 73 patients ages 70 to 89 years (mean age, 76) diagnosed with metastatic colorectal cancer reported that 23% of these older adult patients preferred a collaborative role, 25% an active role and 52% a passive role.60 Other studies have shown that older and less-educated individuals were most likely to prefer passive roles60, 63 while younger, more educated women were most likely to prefer participatory decision making.1, 64-66 Gender may have been a factor in decision making roles according to a study in Britain of older men (58-88 years old, mean 74) diagnosed with prostate cancer, in which the men took a passive role during a treatment discussion but later wanted to revisit the decision-making process.30 Role preferences can also change with time. One study uncovered the dynamic nature of role preferences by 19 showing that cancer patients’ preference for involvement declined as they became sicker.67 Because patients’ preferences for participation in decision making are dynamic, follow-up assessments of patients’ decisional role preferences are important. A recent study of breast cancer patients showed that regardless of race and ethnicity, a higher (more patient-based) rather than lower (more surgeon-based) level of patient participation during decision making was associated with a patient’s decision to have a mastectomy.68 The sample included 23.9% Latina (12.0% low acculturated, 11.9% high acculturated), 27.1% African American, and 48.9% white. In Greece, most women diagnosed with breast cancer (71.1%) preferred a passive role and were found to request less frequent mammography (p<0.001) and/or Papanicolaou tests (p<0.0005) pre-diagnostically.56 These studies demonstrate how decisional control preferences vary and can be associated with actual treatment or health care screening decisions. Health-Related Experience Previous health-related experiences or familiarity with treatment options can influence treatment choice.69, 70 Using grounded theory methods, Berry and colleagues23 uncovered a set of related, meaningful, and influential factors among men who had made recent decisions for localized prostate cancer. Past experience with cancer was the context in which the men in the study deliberated about the current choices. Moreover, the participants were making decisions based on their perceived “best choice” to treat the prostate cancer. A study examining factors that affect older women’s decisions to have regular screening mammograms found that previous negative experience with mammography had considerable influence on their decisions not to continue with a schedule of recommended regular screenings.71 Similarly, a previous negative experience from a colorectal screening procedure 20 (e.g., fecal occult blood or colonoscopy) has been reported as a factor for study participants deciding not to get up-to-date colorectal cancer screenings.72 Perception of the Decision-Making Process The patient’s positive or negative perception of the decision making process could potentially influence cancer treatment decisions. Using content analysis, researchers73 conducted a study exploring the conditions for participation and non-participation in decision making in 362 patients of all diagnoses and conditions in a Swedish medical center. The authors reported that patients participate in health care when information is provided based on their individual needs, when they receive the knowledge needed, and when decisions are made based on their knowledge and needs. Older women diagnosed with breast cancer have also reported higher participation in decision making when they have ample time to exchange information and when their family members are included in the decision-making process. Kreling et al.74 conducted a focus group interview with 34 older women with breast cancer who were from different ethnic backgrounds (29% Black, 53% White, and 18% Latina) to explore the barriers and promoters of chemotherapy use. The researchers noted that there was typically less than adequate time for patient-physician communication prior to decision making—particularly when women of color were the patients—which resulted in a less-than-optimal use of chemotherapy. An evaluation of the patient’s perception of the decision making process would be helpful to health care professionals in promoting future patient participation during decision making. Clearly, according to the research, attention to the patient’s information needs and adequate communication exchange between the provider and the patient are important considerations in increasing patient participation in health care.73, 74 21 Personal Factors Personal factors that could influence cancer treatment decisions include selfdescription, potential treatment outcomes, past experience with cancer, and influential people in the cancer patient’s lives, such as a physician they visited before or other men who shared their beliefs, perspectives, or characteristics.23 Berry and colleagues23 were the first to include a systemic description of “Who I am and what I do” and “Making the best choice for me” as influential aspects of decision making among men with LPC. These descriptive data were obtained from focus groups and individual interviews of 44 men (mean age, 64.8 years) who were within 6 months from the time of diagnosis. The study was exploratory in nature and only 16% of participants were men of color. Denberg and colleagues75 also reported that emotions, misconceptions, and anecdotes influence treatment preferences in patients with prostate cancer. Using semi-structured interviews, Denberg and colleagues explored the personal beliefs and attitudes of 20 men with clinically-localized prostate cancer following their first consultation with a urologist, but before treatments were initiated. Using grounded theory methods, the researchers analyzed the patients’ personal views about prostate cancer and treatment options, emotional reactions to the diagnosis, treatment preferences, information sources, and perceptions of interactions. The researchers found that patients felt the treatment decision-making was made in the setting of intense emotion, a context in which patients were also dealing with fear and uncertainty and had a sense of urgency and needing to make a decision right away.75 The researchers discovered that patients had misunderstood prostatectomy, risks and benefits of other treatment options, and the importance of a second opinion. They also found that patients compared themselves to other men they knew who had been treated for prostate cancer to justify their own treatment decisions. The researchers 22 concluded that patients’ personal factors had an important influence on treatment choice in men with LPC. Patient Contextual Factors The availability of a caregiver or a family member influences treatment decisions 74 and to some extent could lead to decisional conflicts among family members.76, 77 In a crosssectional survey study that achieved a 64% response rate, patients with colorectal cancer ages 65-92 (mean age 75.8, n=67) ranked family preference, family burden, and traveling for treatment, respectively, as the most important factors influencing their treatment decisions.21 Lack of insurance, poor financial status, and geographical barriers have been also reported as important contextual factors that can influence treatment choices.39, 42, 78 Costs, social support, and logistics (such as transportation and lodging) were reported as key issues in treatment selection for adjuvant therapy in older adults with breast, colon, and non-small-cell lung cancers.40 In a study examining the factors that influenced the use of chemotherapy in 34 older adults (≥65 years of age, age range and mean were not reported), Kreling and colleagues74 have identified patient contexts influencing therapy decisions. These contextual factors include secrecy, age, and age-related themes such as functional status and life expectancy, and perceived benefits and side effects of chemotherapy. Depending on the individual combinations of contextual factors for a specific participant, these contextual factors were found to facilitate or inhibit the use of chemotherapy in this group of ethnically diverse older women with breast cancer (18 Caucasians, 10 African-Americans, and 6 Latinas).74 23 Theoretical Frameworks for Patterns of Decisional Role Preferences Physician-based, Shared, and Patient-based TDM There are several frameworks or models of decision making that have been proposed by decision researchers. In a cross-sectional interview study of 467 consecutive patients seen in a general medicine clinic, Mazur and Hickam found that 68% preferred shared decision making, 21.4% preferred physician-based decision making and 10.5% preferred patientbased decision making.79 This study corroborates the decision-making roles described earlier by Degner and Beaton.80 Non-deliberative Delegators, Deliberative Delegators, Non-deliberative Autonomists, and Deliberative Autonomists A typology of decisional roles has been suggested based on a sample of 5,199 older adults from the Wisconsin Longitudinal Study. Flynn and colleagues81 described four prevalent types of role preferences, which include non-deliberative delegators (23%), deliberative delegators (16%), non-deliberative autonomists (11%), and deliberative autonomists (46%). Non-deliberative delegators wanted the physician to make the decision and typically did little deliberation on their own, while deliberative delegators wanted the physician to make the decision but also did a lot of deliberation. Non-deliberative autonomists wanted control over decisions but did little deliberation, while deliberative autonomists wanted personal control and did a lot of deliberation.81 Patterns of Decision Making A well known model of decision making was developed by Degner and Beaton in 1987. “Patterns of Decision Making”80 has been widely used as a theoretical framework for decisional role studies. The framework encompasses the various levels of patient 24 participation in decision making and is patient-centered, directly eliciting participation preferences from the patients’ perspectives. According to the model, four decision-making patterns may occur. A provider-controlled decision-making pattern emerges when patients refuse to become involved in selecting their own treatment, even when urged to do so by the physician. These patients are essentially saying: It’s up to you, Doctor; you’re the expert. On the other hand, a patient-controlled decision-making pattern occurs when patients want to make their own treatment choices.80 When patients indicate a need to discuss available options with their physician and ask for time to think and discuss options prior to making the final decision with the physician at the next visit, a jointly-controlled decision-making pattern occurs. Finally, when patients are incapacitated and unable to make the treatment decision, the family makes treatment decisions for them, resulting in the family-controlled decision-making pattern.80 The description of the patterns of decision making mentioned above provided the initial parameters for the Control Preferences Scale (CPS).82 Table 1 illustrates the various roles patients can play during treatment decision-making. This framework of decisional role patterns was developed based on a 4-year qualitative study into decision-making roles in lifethreatening situations such as cancer.80 The CPS, a measure of decision-making preferences developed by Degner and colleagues,82 identifies these preferences on a series of cards and were utilized to elicit patient preferences for participation during TDM in this study. 25 Table 1. Degner and Beaton’s Pattern of Control (The Control Preferences Scale)80 Active – Patient-Controlled o I prefer to make the final treatment decision (Card A). o I prefer to make the final treatment decision after seriously considering my doctor’s opinion (Card B). Collaborative – Jointly-controlled o I prefer that my doctor and I share responsibility for deciding which treatment is best (Card C). Passive – Provider-controlled o I prefer that my doctor makes the final treatment decision but that he seriously considers my opinion (Card D). o I prefer to leave all treatment decisions to my doctor (Card E). Family-Controlled o Family makes final decisions because the patient is incapacitated (no card) Decisional Role Preferences The patients’ decisional role preferences have been the subject of inquiry among decision making researchers in the past three decades. Initially, researchers have examined the patient’s preferred role, but subsequently they have examined both the preferred and actual role during decision making.3, 9 Preferred and Actual Role in Cancer-related Decision Making Frameworks or models of decision making have emphasized the various roles patients prefer or actualize during the decision making process. Consequently, decision researchers have examined the degree of control patients wanted and achieved during decision making and explored the impact of various preferences for level of participation to decision outcomes such as satisfaction and psychological well-being. A recent systematic review of patient’s decisional preferences in cancer-related decision making has found that patients wanted a more shared or active role versus a less passive role.3 Twenty-two studies involving patients with breast, prostate, colorectal, lung, gynecological, and other cancers were included in the 26 review. The authors concluded that patients wanted more participation than what actually occurred during the decision-making process.3 Additionally, this review validated findings from an earlier systematic review on the same topic, which reported that patients with cancer have large variations in decisional role preferences.1 Interestingly, patients with colorectal cancer were found to have the lowest percentage of active role preference (6%) while patients with prostate cancer (86%) and patients with breast cancer (97%) reported the highest percentage of shared and active role preferences, higher than those with colorectal, gynecological or lung cancer.3 A recent meta-analysis9 of 3491 participants from U.S. and Canadian clinical trials (26% and 74% of the sample, respectively) reported similar findings, that patients wanted more control than what was actualized. Although 49% of the participants preferred a collaborative role, only 34% achieved this role; 25% wanted a passive role but only 36% actually experienced this passive role, resulting in an overall discordance between preferred and actualized roles of 39% (n=1058). Based on the findings of this meta-analysis, Singh and colleagues9 recommended that clinicians assess and individualize their communication approach to cancer patients. Demographic Factors Associated with Decisional Control Preferences in Cancer Patients Sociodemographic factors associated with decisional role preferences may include age, gender, education, ethnicity, employment status, and partner status. In some studies, two or more factors have been associated with specific decisional role preference. Patients younger than 50 years were found in many studies to be significantly more likely to prefer active or collaborative roles during treatment decision making.16, 64, 66, 83-87 Similarly, older adults with cancer were found to prefer a passive role.9, 63, 88-91 Other studies 27 have reported no association between age and role preferences.65, 92-96 Based on these reports, age alone may not be a strong predictor of role preferences, but might be moderated by other sociodemographic factors that influence decisional role preferences. Gender has been reported as a factor influencing decisional role preference. Being male was found to be associated with a passive role preference in two studies.88, 91 A recent meta-analysis reported that more women than men experienced a passive role.9 Education has also been reported as a factor known to be associated with decisional role preferences. Several studies have reported that higher education was found to be associated with a preference for more patient control during decision making in several studies.17, 64, 85, 87, 88 However, other studies have not found an association between educational level and TDM role preferences.92, 95, 96 The small sample sizes (N= ≤150) of these latter studies may have limited more meaningful association analyses. In terms of employment or partner status and role preferences, one study reported that being employed is associated with active role preference,85 while another study reported that married men and women were more likely to prefer a passive role.86 Ethnicity may also be a factor associated with decisional role preferences. East Asians,97, 98 Hispanics, and African Americans were found to be more likely than Caucasian to prefer passive or collaborative than active roles in TDM.57 Two studies have reported specifically that they found no association between various sociodemographic variables and TDM role preferences.60, 99 More studies are needed to better understand the relationship between ethnicity and role preferences. As discussed above, several studies have shown that certain sociodemographic factors are associated with decisional control preferences in cancer patient populations. However, 28 there is a high variability in patients’ decisional role preferences, and sociodemographic factors only account for a small percentage of the variance in role preferences. Thus, these associations must be interpreted with great caution, and an individualized assessment of patient preferences should be investigated across the continuum of care. Priority of Information Needs in Cancer Patients Information seeking about the disease, its prognosis and treatment remains a major area of need for individuals with cancer.100 In order to truly help patients make autonomous decisions, health care professionals have a responsibility to provide accurate, timely, and meaningful information to patients. Since health care resources are limited, prioritizing patients’ information needs is an important step towards efficiency. Oncology nurses play a critical role in providing individual information to patients with cancer. The measurement and prioritization of the patient’s information needs in nursing care potentially offers the following advantages: 66, 89, 101 directs the attention of nurses to the highest information needs guides nurses to prioritize patient teaching and information giving saves time and enhances the quality of information that patients will receive provides relevant information to patients at specific points of their disease and recovery trajectory makes nurse-patient encounters more meaningful lowers the psychological distress associated with treatment decision-making, and helps patients assume a more active role in decision making generally. Table 2 outlines a summary of studies regarding information priorities of patients with cancer. Despite recent efforts to prioritize patients’ information needs, the assessment of 29 patients’ priorities for these needs and mapping them over time remain a logistical issue for most health care professionals. 30 Table 2. Summary Table of Information Needs Priorities in Cancer Patients Reference Cassileth et al.102 Sample size, age range, mean age 256 patients, age range not reported, 55.5 years Davison et al.103 74 patients with their partners, 4079 years, 62.2 years Wallberg et al.87 201 patients, age range and mean not reported; 44% <50 years, 36% 51–65 years, 20% ≥66 years 150 patients; 32-84 years, 54.8 years Luker et al.104 Methods: design, sample, sampling, setting; data collection Cross-sectional, patients with various types of cancer diagnosed within the past 10 months, consecutive sampling from hematology/oncology, radiation therapy, and inpatient oncology unit; survey investigator-developed ISQ Quasi-experimental, one-group, pretest/post-test, patients with prostate cancer who were diagnosed recently and had their initial treatment consultation, convenience sampling from an outpatient prostate center; survey using the INQ Cross-sectional, women with breast cancer diagnosed in the past 18 months, consecutive sampling from an outpatient breast cancer clinic; structured interview using the INQ Cross-sectional, newly diagnosed breast cancer patients with an average 2.5 weeks since diagnosis, consecutive sampling from a consultant’s list in a large teaching hospital; structured interview; INQ Top three patient priorities for information related to TDM 1. Side effects of therapy 2. Goals of therapy 3. Disease 1. Likelihood of cure 2. Stage of disease 3. Side effects of therapy 1. Likelihood of cure 2. Stage of disease 3. Risks and benefits of various treatment 1. Likelihood of cure 2. Stage of disease 3. Risks and benefits of various treatments 30 31 Table 2 continued Reference Beaver et al.99 Stewart et al.93 Sample size, age range, mean age 42 patients, 43-83 years, 66.6 years 105 patients, 21-87 years, 55.8 years Methods: design, sample, sampling, setting; data collection Cross-sectional, primarily male patients with known first-time colorectal cancer, convenience sampling from general practitioner referrals and a small number of inpatient referrals at a large university teaching hospital; interview schedule; INQ Cross-sectional, women with ovarian cancer, including those in remission, with metastasis or distant metastasis, consecutive sampling from a university general hospital and at a comprehensive cancer center; self-report questionnaire; investigator-developed 5-point Likert type, investigator-developed 43-item INQ Top three patient priorities for information related to TDM 1. Likelihood of cure 2. Stage of disease 3. Risks and benefits of various treatments 1. Status and nature of the disease 2. Various treatment and success rates 3. Self-care and empowerment issues 31 32 Table 2 continued Reference Vogel et al.105 Sample size, age range, mean age 135 patients, 19-75 years, 53.9 years Methods: design, sample, sampling, setting; data collection Longitudinal, women with newly diagnosed breast cancer, consecutive sampling from two breast cancer centers; mailed questionnaires; investigatordeveloped 5-point Likert-type information needs questionnaire with eight items Top three patient priorities for information related to TDM At baseline: 1. Treatment 2. Diagnosis 3. Prognosis/medication side effects/aftercare (equal scores) At 3 months: 1. Treatment 2. Medication and side effects 3. Aftercare At 6 months: 1. Aftercare 2. Treatment 3. Prognosis/medication side effects/examination & medical tests (equal scores) 32 33 Table 2 continued Reference Butow et al.67 Sample size, age range, mean age 80 patients, 18-87 years, 51 years Methods: design, sample, sampling, Top three patient priorities for setting; data collection information related to TDM Longitudinal, patients with various types Pre-consultation: of newly diagnosed cancer comprising 1. Information on what is happening mostly women (75%), consecutive to the cancer sampling from two participating 2. Information on the likely future of oncologists at a university hospital; survey the illness questionnaires using ISQ 3. Information about the illness Immediately after consultation: 1. Information on support in the form of reassurance that they would be “looked after” 2. Information on support in terms of reassurance and hope 3. Information on support in terms of a chance to talk about worries and fears 3-6 months after first visit (N=40) 1. Information on the likely future of the cancer 2. Information on what is happening to the cancer 3. Information on support in terms of reassurance and hope 33 34 Table 2 continued Reference Bilodeau & Degner89 Sample size, age range, mean age 74 patients, 18-83 years, 57.5 years Davison, Degner, & Morgan106 57 patients, age range not reported, mean age was 71 years Davison et al.19 162 patients, age range not reported, mean age was 62.4 years Davidson, Brundage, & FeldmanStewart107 22 patients, age range not reported, mean age was 65 Methods: design, sample, sampling, setting; data collection Cross-sectional, women with breast cancer diagnosed in the past 6 months, convenience sampling from two tertiary referral centers; survey questionnaires using INQ Cross-sectional, men with prostate cancer diagnosed in the past 6 months, convenience sampling from a community urology clinic; survey questionnaires; INQ Cross-sectional, men diagnosed with prostate cancer recently and just had their initial urologic treatment consultation, consecutive sampling from a tertiary hospital; computerized version of INQ Cross-sectional, newly diagnosed lung cancer patients with a median time of 8.3 months from diagnosis, consecutive sampling from a gynecological service of a large teaching hospital and oncology unit; survey questionnaires; investigatordeveloped questionnaire on information priorities Top three patient priorities for information related to TDM 1. Stage of disease 2. Likelihood of cure 3. Risks and benefits of various treatments 1. Likelihood of cure 2. Stage of disease 3. Risks and benefits of treatments 1. Likelihood of cure 2. Disease stage 3. Risks and benefits of various treatments 1. Treatment 2. Survival 3. Side effects of therapy 34 35 Table 2 continued Reference Beaver & Booth108 Sample size, age range, mean age 53 patients, 24-82 years, 55 years Gopal et al.109 100 patients, 32-60 years, 45.09 years Li et al.110 374 patients, 31-85 years, 55.4 years Degner et al.66 1,012 patients, age range not reported, 58.25 years Methods: design, sample, sampling, Top three patient priorities for setting; data collection information related to TDM Cross-section, newly diagnosed 1. Likelihood of cure gynecological cancer patients; consecutive 2. Stage of disease sampling from outpatient clinics of a large 3. Side effects of therapy teaching hospital; structured interviews using INQ Cross-sectional, women who were newly 1. Likelihood of cure diagnosed with breast cancer, between 3 2. Sexual attractiveness and 4 months from diagnosis, 3. Stage of disease convenience sampling from two major hospitals; survey questionnaire using INQ Cross-sectional, women diagnosed with 1. Likelihood of cure breast cancer with a mean time of 19.7 2. Stage of disease months since diagnosis, convenience 3. Risks and benefits of various sampling from a large regional hospital; therapies survey questionnaires using INQ-Chinese version Cross-sectional, women diagnosed with 1. Likelihood of cure breast cancer with a median time of 2.5 2. Stage of disease years since diagnosis, consecutive 3. Risks and benefits of various sampling from 2 tertiary oncology referral therapies clinics; survey, nurse-administered questionnaire; INQ 35 36 Table 2 continued Reference Sample size, age range, mean age 105 patients, 35-80 years, 56.02 years Methods: design, sample, sampling, setting; data collection Cross-sectional, women diagnosed with breast cancer assessed at time of diagnosis and follow-up (mean time of 21 months since diagnosis), consecutive sampling from a consultant’s list in a large teaching hospital; structured interview; INQ Mistry et al.112 187 patients, 24-91 years, 58.8 years Almyroudi et al.56 329 patients, age range not reported, 59.5 years Cross-sectional, men and women diagnosed with various types of cancer at pre- and post-treatment stage, convenience sampling from outpatient oncology clinics; survey questionnaires using investigator-developed 35-item Likert-type information needs categories Cross-sectional, breast cancer patients with a mean of 43.37 months disease duration, consecutive sampling from a large university general hospital; semistructured interview using ISQ Luker et al.111 Top three patient priorities for information related to TDM At diagnosis: 1. Likelihood of cure 2. Stage of disease 3. Risks and benefits of therapy At follow-up: 1. Likelihood of cure 2. Family risk 3. Stage of disease 1. Information related to cancer domain 2. Information related to prognosis domain 3. Information related to rehabilitation domain 1. Information on investigative tests that must be done, and when 2. Diagnosis of cancer 3. Exact treatment plan the doctor has decided 36 37 Table 2 continued Reference Sample size, age range, mean age 731 patients, 20-92 years, 61 years Methods: design, sample, sampling, Top three patient priorities for setting; data collection information related to TDM Longitudinal, patients who had been 1. Information on likelihood of how Hawkins et al.113 diagnosed with breast, lung, the treatment will work genitourinary, hematologic, 2. Information on how to relieve side gastrointestinal, or head and neck cancer effects and whose treatment plan included 3. Information on how to ease the chemotherapy or radiation therapy, family’s stress median time from diagnosis to baseline interview was 39 days, convenience sampling from a large university-based cancer center; investigator-developed 5point Likert-type information needs questionnaire 15 patients, 58-86 Cross-sectional, patients newly diagnosed 1. Tests/treatment Andreassen et years, 69 years with esophageal cancer in the past 2-3 2. Health care professional’s al.114 weeks, consecutive sampling from an competence outpatient clinic; survey using 3. Self-care investigator-developed 5-point Likert-type information needs questionnaire Abbreviations: ISQ – Information Style Questionnaire;102 INQ – Information Needs Questionnaire101 37 38 Demographic Factors Associated with Information Needs Priorities in Cancer Patients Sociodemographic factors have been examined for their association with patients’ information needs priorities. One study reported that patients’ preference for more information relating to prognosis was associated with being male.60 Two studies66, 87 reported that information about sexuality and physical attractiveness was more important to younger women (≤ 50 years) than older women (≥ 50 years) (p<.001). Luker and colleagues also reported similar findings.104 Similarly, Davison and colleagues103 reported that young men (< 65 years) ranked information on sexuality as more important than older men (≥65 years). Moreover, older women (≥60 years age group) rated information pertaining to social life as more important than did younger women (p = 0.03).104 Bilodeau and Degner89 reported higher age (specifically within the 65-85 year-old category) to be significantly associated with a higher ranking for self-care information (p ≤0.02). One study reported that women with less than a high school education wanted more information on self-care (p = <0.02) than did women with higher education,89 while another study reported that women with less than high school education wanted relatively more information on likelihood of cure.87 However, two other studies found no difference in information needs by educational level.66, 104 Davison, Degner and Morgan106 reported that single men ranked information on selfcare significantly higher than did married men (p<0.001). One study reported no association between age, level of education or partner status and information needs priorities.19 39 Conclusion Treatment decision making in cancer involves information giving, processing of information, weighing of risks and benefits of therapy, and patient participation or nonparticipation during the decision making process. This review highlighted the relevant physician and patient factors that could influence treatment decisions in cancer patients. Physician-related factors include physician’s beliefs, attitudes, and values; medical expertise and specialty; patient’s medical and clinical conditions; communication style and medical literature. Influential patient-related decision factors include patient’s beliefs and values; ethnicity; decisional role preferences; personal factors; context; and perception of the decision-making process. When patient and physician decisional factors differ in the setting of clinical uncertainty, it is critical that adequate time is allotted for effective communication between physicians and patients to account for the influential personal and contextual factors from both perspectives. Effective communication exchange is essential for the achievement of individualized treatment decisions. Frameworks or models of decision making have essentially focused on the patient’s roles during the decision-making process. There is a growing trend among patients of wanting to be informed and involved with the TDM process, as evidenced by the high percentage of patients who prefer a shared and active role over a passive one. Several decision-making studies have been conducted in breast and prostate cancer populations that already bear this trend out. However, more such studies are now being conducted in colorectal, lung and gynecological cancer patient populations. Unfortunately, there is still a paucity of decision-making research in hematologic and other solid organ malignancies. 40 In the area of information giving, this review reveals that decision researchers consistently find that there is a discernible priority of information needs among cancer patients, needs that include prognosis, diagnosis, treatments, side effects, and self-care. Although clear patterns emerge in this research, there are still huge variations in patients’ reported priorities of information needs. Moreover, it has been found that preferences and priorities do change over time for individuals, depending on stage of the disease and other factors. Interestingly, sociodemographic factors do not always factor into patient preferences for TDM participation and information needs. Therefore, it is critical that health care professionals assess their patients’ individual preferences for TDM participation and priority of information needs across the entire care continuum. Finally, since treatment decisionmaking primarily involves the physician and the patient, it is essential for decision researchers to examine both patient-related and physician-related factors simultaneously in order to understand how the interaction of these two factor group ultimately impacts treatment choice. 41 References 1. Gaston CM, Mitchell G. Information giving and decision-making in patients with advanced cancer: a systematic review. Soc Sci Med. 2005;61(10):2252-2264. 2. Rutten LJ, Arora NK, Bakos AD, et al. Information needs and sources of information among cancer patients: a systematic review of research (1980-2003). Patient Educ Couns. 2005;57(3):250-261. 3. Tariman JD, Berry DL, Cochrane B, et al. Preferred and actual participation roles during health care decision making in persons with cancer: a systematic review. Ann Oncol. 2010;21(6):1145-1151. 4. Husson O, Mols F, van de Poll-Franse LV. The relation between information provision and health-related quality of life, anxiety and depression among cancer survivors: a systematic review. Ann Oncol. Epub ahead of print. 5. Volk RJ, Hawley ST, Kneuper S, et al. Trials of decision aids for prostate cancer screening: a systematic review. Am J Prev Med. 2007;33(5):428-434. 6. Chouliara Z, Kearney N, Stott D, et al. Perceptions of older people with cancer of information, decision making and treatment: a systematic review of selected literature. Ann Oncol. 2004;15(11):1596-1602. 7. O'Connor AM, Stacey D, Rovner D, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2001(3):CD001431. 8. Walsh MC, Trentham-Dietz A, Schroepfer TA, et al. Cancer information sources used by patients to inform and influence treatment decisions. J Health Commun. 2010;15(4):445-463. 42 9. Singh JA, Sloan JA, Atherton PJ, et al. Preferred roles in treatment decision making among patients with cancer: a pooled analysis of studies using the Control Preferences Scale. Am J Manag Care. 2010;16(9):688-696. 10. Weinfurt KP. Outcomes research related to patient decision making in oncology. Clin Ther. 2003;25(2):671-683. 11. Feldmann EG. Editorial: Paternalism is out. J Pharm Sci. 1973;62(10):1. 12. Kao CC. Maturity and paternalism in health care. Ethics Sci Med. 1976;3(3):179-186. 13. Schain WS. Patients' rights in decision making: the case for personalism versus paternalism in health care. Cancer. 1991;46(4 Suppl):1035-1041. 14. Frosch DL, Kaplan RM. Shared decision making in clinical medicine: past research and future directions. Am J Prev Med. 1999;17(4):285-294. 15. Ernst J, Kuhnt S, Schwarzer A, et al. The desire for shared decision making among patients with solid and hematological cancer. Psychooncology. 2011;20(2):186-193. 16. Caldon LJ, Walters SJ, Reed MW. Changing trends in the decision-making preferences of women with early breast cancer. Br J Surg. 2008;95(3):312-318. 17. Janz NK, Wren PA, Copeland LA, et al. Patient-physician concordance: preferences, perceptions, and factors influencing the breast cancer surgical decision. J Clin Oncol. 2004;22(15):3091-3098. 18. Davison BJ, Goldenberg SL, Gleave ME, et al. Provision of individualized information to men and their partners to facilitate treatment decision making in prostate cancer. Oncol Nurs Forum. 2003;30(1):107-114. 43 19. Davison BJ, Goldenberg SL, Wiens KP, et al. Comparing a generic and individualized information decision support intervention for men newly diagnosed with localized prostate cancer. Cancer Nurs. 2007;30(5):E7-E15. 20. Hotta K, Kiura K, Takigawa N, et al. Desire for information and involvement in treatment decisions: lung cancer patients' preferences and their physicians' perceptions: results from Okayama Lung Cancer Study Group Trial 0705. J Thorac Oncol. 2010;5(10):1668-1672. 21. Kutner JS, Vu KO, Prindiville SA, et al. Patient age and cancer treatment decisions. Patient and physician views. Cancer Pract. 2000;8(3):114-119. 22. Berry DL, Maliski SL, Ellis WJ. Qualitative research techniques. In: Penson DF, Wei JT, eds. Clinical Research Methods for Surgeons. Totowa, NJ: Human Press, Inc; 2006:297-310. 23. Berry DL, Ellis WJ, Woods NF, et al. Treatment decision-making by men with localized prostate cancer: the influence of personal factors. Urol Oncol. 2003;21(2):93-100. 24. Mandelblatt JS, Sheppard VB, Hurria A, et al. Breast cancer adjuvant chemotherapy decisions in older women: the role of patient preference and interactions with physicians. J Clin Oncol. 2010;28(19):3146-3153. 25. Wedding U, Pientka L, Hoffken K. Quality-of-life in elderly patients with cancer: a short review. Eur J Cancer. 2007;43(15):2203-2210. 44 26. Kvam AK, Fayers P, Hjermstad M, et al. Health-related quality of life assessment in randomised controlled trials in multiple myeloma: a critical review of methodology and impact on treatment recommendations. Eur J Haematol. 2009;83(4):279-289. 27. Bouchardy C, Rapiti E, Blagojevic S, et al. Older female cancer patients: importance, causes, and consequences of undertreatment. J Clin Oncol. 2007;25(14):1858-1869. 28. Freedman TG. 'The doctor knows best' revisited: physician perspectives. Psychooncology. 2002;11(4):327-335. 29. Elit L, Charles C, Gold I, et al. Women's perceptions about treatment decision making for ovarian cancer. Gynecol Oncol. 2003;88(2):89-95. 30. Cohen H, Britten N. Who decides about prostate cancer treatment? A qualitative study. Fam Pract. 2003;20(6):724-729. 31. Sekeres MA, Stone RM, Zahrieh D, et al. Decision-making and quality of life in older adults with acute myeloid leukemia or advanced myelodysplastic syndrome. Leukemia. 2004;18(4):809-816. 32. Ravdin PM, Siminoff IA, Harvey JA. Survey of breast cancer patients concerning their knowledge and expectations of adjuvant therapy. J Clin Oncol. 1998;16(2):515521. 33. O'Toole E, Step MM, Engelhardt K, et al. The role of primary care physicians in advanced cancer care: perspectives of older patients and their oncologists. J Am Geriatr Soc. 2009;57 Suppl 2:S265-268. 45 34. Lyman GH, Giuliano AE, Somerfield MR, et al. American Society of Clinical Oncology guideline recommendations for sentinel lymph node biopsy in early-stage breast cancer. J Clin Oncol. 2005;23(30):7703-7720. 35. Ng AK, Li S, Neuberg D, Silver B, et al. Factors influencing treatment recommendations in early-stage Hodgkin's disease: a survey of physicians. Ann Oncol. 2004;15(2):261-269. 36. Fowler FJ, Jr., McNaughton Collins M, Albertsen PC, et al. Comparison of recommendations by urologists and radiation oncologists for treatment of clinically localized prostate cancer. JAMA;283(24):3217-3222. 37. de Jong D, Aleman BM, Taal BG, et al. Controversies and consensus in the diagnosis, work-up and treatment of gastric lymphoma: an international survey. Ann Oncol. 1999;10(3):275-280. 38. Repetto L, Comandini D, Mammoliti S. Life expectancy, comorbidity and quality of life: the treatment equation in the older cancer patients. Crit Rev Oncol Hematol. 2001;37(2):147-152. 39. Schrag D, Cramer LD, Bach PB, et al. Age and adjuvant chemotherapy use after surgery for stage III colon cancer. J Natl Cancer Inst. 2001;93(11):850-857. 40. Muss HB, Biganzoli L, Sargent DJ, et al. Adjuvant therapy in the elderly: making the right decision. J Clin Oncol. 2007;25(14):1870-1875. 41. Hurria A, Leung D, Trainor K, et al. Factors influencing treatment patterns of breast cancer patients age 75 and older. Crit Rev Oncol Hematol. 2003;46(2):121-126. 46 42. Bailey C, Corner J, Addington-Hall J, et al. Treatment decisions in older patients with colorectal cancer: the role of age and multidimensional function. Eur J Cancer Care (Engl). 2003;12(3):257-262. 43. Klepin HD, Hurd DD. Autologous transplantation in elderly patients with multiple myeloma: are we asking the right questions? Bone Marrow Transplant. 2006;38(9):585-592. 44. Giordano SH, Duan Z, Kuo YF, et al. Use and outcomes of adjuvant chemotherapy in older women with breast cancer. J Clin Oncol. 2006;24(18):2750-2756. 45. Balducci L. Management of cancer in the elderly. Oncology (Williston Park). 2006;20(2):135-143; discussion 144, 146, 151-132. 46. Step MM, Siminoff LA, Rose JH. Differences in oncologist communication across age groups and contributions to adjuvant decision outcomes. J Am Geriatr Soc. 2009;57 Suppl 2:S279-282. 47. Sargent D. What constitutes reasonable evidence of efficacy and effectiveness to guide oncology treatment decisions? Oncologist. 2010;15 Suppl 1:19-23. 48. Fried TR, Bradley EH, Towle VR, et al. Understanding the treatment preferences of seriously ill patients. N Engl J Med. 2002;346(14):1061-1066. 49. Knight SJ, Latini DM. Sexual side effects and prostate cancer treatment decisions: patient information needs and preferences. Cancer J. 2009;15(1):41-44. 50. Martin VC, Roberto KA. Assessing the stability of values and health care preferences of older adults: A long-term comparison. J Gerontol Nurs. 2006;32(11):23-33. 47 51. Langer AS. Side effects, quality-of-life issues, and trade-offs: the patient perspective. J Natl Cancer Inst Monogr. 2001(30):125-129. 52. O'Rourke ME. Choose wisely: therapeutic decisions and quality of life in patients with prostate cancer. Clin J Oncol Nurs. 2007;11(3):401-408. 53. Repetto L, Ausili-Cefaro G, Gallo C, et al. Quality of life in elderly cancer patients. Ann Oncol. 2001;12 Suppl 3:S49-52. 54. Husain LS, Collins K, Reed M, et al. Choices in cancer treatment: a qualitative study of the older women's (>70 years) perspective. Psychooncology. 2008;17(4):410-416. 55. Pasetto LM, Falci C, Compostella A, et al. Quality of life in elderly cancer patients. Eur J Cancer. 2007;43(10):1508-1513. 56. Almyroudi A, Degner LF, Paika V, et al. Decision-making preferences and information needs among Greek breast cancer patients. Psychooncology. 2010; Epub ahead of print. 57. Blackhall LJ, Murphy ST, Frank G, et al. Ethnicity and attitudes toward patient autonomy. JAMA. 1995;274(10):820-825. 58. Friedman MM, Bowden VR, Jones EG. Family nursing research theory and practice. 5th ed. Upper Saddle, NJ: Prentice Hall; 2003. 59. Doolen J, York NL. Cultural differences with end-of-life care in the critical care unit. Dimens Crit Care Nurs. 2007;26(5):194-198. 60. Elkin EB, Kim SH, Casper ES, et al. Desire for information and involvement in treatment decisions: elderly cancer patients' preferences and their physicians' perceptions. J Clin Oncol. 2007;25(33):5275-5280. 48 61. Nease RFJ, Brooks WB. Patient desire for information and decision making in health care decisions: the Autonomy Preference Index and the Health Opinion Survey. J Gen Intern Med. 1995;10(11):593-600. 62. Robinson A, Thomson R. Variability in patient preferences for participating in medical decision making: implication for the use of decision support tools. Qual Health Care. 2001;10 Suppl 1:i34-38. 63. Deber RB, Kraetschmer N, Urowitz S, et al. Do people want to be autonomous patients? Preferred roles in treatment decision-making in several patient populations. Health Expect. 2007;10(3):248-258. 64. Ryan J, Sysko J. The contingency of patient preferences for involvement in health decision making. Health Care Manage Rev. 2007;32(1):30-36. 65. Bruera E, Sweeney C, Calder K, et al. Patient preferences versus physician perceptions of treatment decisions in cancer care. J Clin Oncol. 2001;19(11):28832885. 66. Degner LF, Kristjanson LJ, Bowman D, et al. Information needs and decisional preferences in women with breast cancer. JAMA. 1997;277(18):1485-1492. 67. Butow PN, Maclean M, Dunn SM, et al. The dynamics of change: cancer patients' preferences for information, involvement and support. Ann Oncol. 1997;8(9):857863. 68. Hawley ST, Griggs JJ, Hamilton AS, et al. Decision involvement and receipt of mastectomy among racially and ethnically diverse breast cancer patients. J Natl Cancer Inst. 2009;101(19):1337-1347. 49 69. Mazur DJ, Merz JF. How older patients' treatment preferences are influenced by disclosures about therapeutic uncertainty: surgery versus expectant management for localized prostate cancer. J Am Geriatr Soc. 1996;44(8):934-937. 70. Kelly-Powell ML. Personalizing choices: patients' experiences with making treatment decisions. Res Nurs Health. 1997;20(3):219-227. 71. Schonberg MA, McCarthy EP, York M, et al. Factors influencing elderly women's mammography screening decisions: implications for counseling. BMC Geriatr. 2007;7:26. 72. Janz NK, Lakhani I, Vijan S, et al. Determinants of colorectal cancer screening use, attempts, and non-use. Prev Med. 2007;44(5):452-458. 73. Eldh AC, Ekman I, Ehnfors M. Conditions for patient participation and nonparticipation in health care. Nurs Ethics. 2006;13(5):503-514. 74. Kreling B, Figueiredo MI, Sheppard VL, et al. A qualitative study of factors affecting chemotherapy use in older women with breast cancer: barriers, promoters, and implications for intervention. Psychooncology. 2006;15(12):1065-1076. 75. Denberg TD, Melhado TV, Steiner JF. Patient treatment preferences in localized prostate carcinoma: The influence of emotion, misconception, and anecdote. Cancer. 2006;107(3):620-630. 76. Zhang AY, Siminoff LA. The role of the family in treatment decision making by patients with cancer. Oncol Nurs Forum. 2003;30(6):1022-1028. 77. Schafer C, Putnik K, Dietl B, et al. Medical decision-making of the patient in the context of the family: results of a survey. Support Care Cancer. 2006;14(9):952-959. 50 78. Mandelblatt JS, Yabroff KR, Kerner JF. Equitable access to cancer services: A review of barriers to quality care. Cancer. 1999;86(11):2378-2390. 79. Mazur DJ, Hickam DH. Patients' preferences for risk disclosure and role in decision making for invasive medical procedures. J Gen Intern Med. 1997;12(2):114-117. 80. Degner LF, Beaton JI. Life death decisions in health care. New York: Hemisphere Publishing; 1987. 81. Flynn KE, Smith MA, Vanness D. A typology of preferences for participation in healthcare decision making. Soc Sci Med. 2006;63(5):1158-1169. 82. Degner LF, Sloan JA, Venkatesh P. The Control Preferences Scale. Can J Nurs Res. 1997;29(3):21-43. 83. Hawley ST, Lantz PM, Janz NK, et al. Factors associated with patient involvement in surgical treatment decision making for breast cancer. Patient Educ Couns. 2007;65(3):387-395. 84. Salkeld G, Solomon M, Short L, et al. A matter of trust--patient's views on decisionmaking in colorectal cancer. Health Expect. 2004;7(2):104-114. 85. Rothenbacher D, Lutz MP, Porzsolt F. Treatment decisions in palliative cancer care: patients' preferences for involvement and doctors' knowledge about it. Eur J Cancer. 1997;33(8):1184-1189. 86. Blanchard CG, Labrecque MS, Ruckdeschel JC, et al. Information and decisionmaking preferences of hospitalized adult cancer patients. Soc Sci Med. 1988;27(11):1139-1145. 51 87. Wallberg B, Michelson H, Nystedt M, et al. Information needs and preferences for participation in treatment decisions among Swedish breast cancer patients. Acta Oncol. 2000;39(4):467-476. 88. Degner LF, Sloan JA. Decision making during serious illness: what role do patients really want to play? J Clin Epidemiol. 1992;45(9):941-950. 89. Bilodeau BA, Degner LF. Information needs, sources of information, and decisional roles in women with breast cancer. Oncol Nurs Forum. 1996;23(4):691-696. 90. Fischer M, Visser A, Voerman B, et al. Treatment decision making in prostate cancer: patients' participation in complex decisions. Patient Educ Couns. 2006;63(3):308313. 91. Stiggelbout AM, Kiebert GM. A role for the sick role. Patient preferences regarding information and participation in clinical decision-making. CMAJ. 1997;157(4):383389. 92. Ramfelt E, Lutzen K, Nordstrom G. Treatment decision-making in a group of patients with colo-rectal cancer before surgery and a one-year follow-up. Eur J Cancer Care (Engl). 2005;14(4):327-335. 93. Stewart DE, Wong F, Cheung AM, et al. Information needs and decisional preferences among women with ovarian cancer. Gynecol Oncol. 2000;77(3):357-361. 94. Bruera E, Willey JS, Palmer JL, et al. Treatment decisions for breast carcinoma: patient preferences and physician perceptions. Cancer. 2002;94(7):2076-2080. 95. Beaver K, Luker KA, Owens RG, et al. Treatment decision making in women newly diagnosed with breast cancer. Cancer Nurs. 1996;19(1):8-19. 52 96. Davison BJ, Parker PA, Goldenberg SL. Patients' preferences for communicating a prostate cancer diagnosis and participating in medical decision-making. BJU Int. 2004;93(1):47-51. 97. Lee A, Wu HY. Diagnosis disclosure in cancer patients--when the family says "no!". Singapore Med J. 2002;43(10):533-538. 98. Back MF, Huak CY. Family centered decision making and non-disclosure of diagnosis in a South Asian oncology practice. Psycho-Oncology. 2005;14:1052-1059. 99. Beaver K, Bogg J, Luker KA. Decision-making role preferences and information needs: a comparison of colorectal and breast cancer. Health Expect. 1999;2(4):266276. 100. Nagler RH, Gray SW, Romantan A, et al. Differences in information seeking among breast, prostate, and colorectal cancer patients: Results from a population-based survey. Patient Educ Couns. 2010; 81 Suppl:S54-62. 101. Degner LF, Davison BJ, Sloan JA, et al. Development of a scale to measure information needs in cancer care. J Nurs Meas. 1998;6(2):137-153. 102. Cassileth BR, Zupkis RV, Sutton-Smith K, et al. Information and participation preferences among cancer patients. Ann Intern Med. 1980;92(6):832-836. 103. Davison BJ, Gleave ME, Goldenberg SL, et al. Assessing information and decision preferences of men with prostate cancer and their partners. Cancer Nurs. 2002;25(1):42-49. 104. Luker KA, Beaver K, Leinster SJ, et al. The information needs of women newly diagnosed with breast cancer. J Adv Nurs. 1995;22(1):134-141. 53 105. Vogel BA, Bengel J, Helmes AW. Information and decision making: Patients' needs and experiences in the course of breast cancer treatment. Patient Educ Couns. 2008;71(1):79-85. 106. Davison BJ, Degner LF, Morgan TR. Information and decision-making preferences of men with prostate cancer. Oncol Nurs Forum. 1995;22(9):1401-1408. 107. Davidson JR, Brundage MD, Feldman-Stewart D. Lung cancer treatment decisions: patients' desires for participation and information. Psychooncology. 1999;8(6):511520. 108. Beaver K, Booth K. Information needs and decision-making preferences: comparing findings for gynaecological, breast and colorectal cancer. Eur J Oncol Nurs. 2007;11(5):409-416. 109. Gopal RL, Beaver K, Barnett T, et al. A comparison of the information needs of women newly diagnosed with breast cancer in Malaysia and the United Kingdom. Cancer Nurs. 2005;28(2):132-140. 110. Li PWC, So WKW, Fong DYT, et al. The information needs of breast cancer patients in Hongkong and their level of satisfaction with the provision of information. Cancer Nurs. 2011;34(1):49-57. 111. Luker KA, Beaver K, Leinster SJ, et al. Information needs and sources of information for women with breast cancer: a follow-up study. J Adv Nurs. 1996;23(3):487-495. 112. Mistry A, Wilson S, Priestman T, et al. How do the information needs of cancer patients differ at different stages of the cancer journey? A cross-sectional survey. JRSM Short Rep. 2010;1(4):30. 54 113. Hawkins NA, Pollack LA, Leadbetter S, et al. Informational needs of patients and perceived adequacy of information available before and after treatment of cancer. J Psychosoc Oncol. 2008;26(2):1-16. 114. Andreassen S, Randers I, Naslund E, et al. Information needs following a diagnosis of oesophageal cancer; self-perceived information needs of patients and family members compared with the perceptions of healthcare professionals: a pilot study. Eur J Cancer Care (Engl). 2007;16(3):277-285. 55 CHAPTER III Patient and Physician Perspectives on Treatment Decision Making in Older Adults Newly Diagnosed with Myeloma Introduction Multiple myeloma (MM) is a cancer of the plasma cells. It affects primarily the elderly, with the highest incidence occurring at the sixth through the eighth decade of life.1 In 2010, there were approximately 20,180 new cases diagnosed in the U.S., making it the second most common hematologic malignancy after non-Hodgkin’s lymphoma.2 The overall annual incidence rate of myeloma in the United States from 1973-2005, age-adjusted to the 2009 population, was 11.0 and 4.3 per 100,000 person-years for blacks and whites, respectively.3 MM has worldwide incidence rates from 0.4 to 5 persons per every 100,000 on a given year, with rates being higher in Western than in Asian countries.4, 5 MM is not curable; however, there are many effective treatments available that can extend patient survival with relatively good overall quality of life.6 Randomized controlled trials (RCTs) have compared conventional chemotherapies with high-dose chemotherapies followed by an autologous hematopoietic stem cell transplantation (HSCT). The results of these trials show equivocal results in terms of overall survival, though they do show that the chemotherapy plus autologous HSCT combination treatment offers longer progression-free survival.7, 8 Autologous HSCT is still widely accepted as a treatment option for myeloma patients <65 years of age, yet it is now being challenged through historical control studies and randomized controlled trials comparing outcomes from intensive therapy versus non-intensive therapy using novel drugs.9-11 RCTs comparing the use of novel treatment agents with or without conventional chemotherapies 56 against conventional chemotherapies are underway. However, to date there are no RCTs comparing high-dose chemotherapy followed by HSCT with novel therapies.11 Older adults newly diagnosed with multiple myeloma have multiple treatment options available, each with potentially different clinical outcomes and risk/benefit trade-offs. Evidence-based treatment guidelines developed by the National Comprehensive Cancer Network (NCCN), an organization of 21 leading comprehensive cancer centers in the U.S., do not identify one treatment as unequivocally superior to all alternatives for a given set of conditions, based on available data.12 Myeloma treatments also come in various forms, routes, and intensities, including oral, IV, and high-dose chemotherapies or reduced intensity conditioning followed by HSCT. These chemotherapeutic regimens have different patient adherence and completion rates. Other factors, such as direct cost to the patient (co-pays and deductibles) and insurance coverage status, may also influence treatment considerations. It is unclear how these variables ultimately influence actual treatment choices in older adults newly diagnosed with myeloma. Given the lack of one recognized “best” medical therapy, patients are in a position to choose one or more treatments among the many available options. This notion of patient’s treatment selection comes with the assumption that physicians offer options to their patients. Whether or not patients actually participate in their treatment decision, they are still vested in the outcome. In other cancer diagnoses where adults have multiple treatment choices, there is strong evidence that personal factors and preferences are quite influential in determining how patients arrive at a final treatment decision.13, 14 Similarly, physician preferences and values have also been found to be influential in actual treatment choices.15-17 57 The clinical uncertainties in patients newly diagnosed with myeloma can be largely attributed to the existence of multiple possible outcomes, each with unknown probabilities. Adding to the complexity is the presence of new and emerging scientific data and difficulties for physicians in applying clinical trial data to particular patients. Moreover, advances in myeloma genomics are beginning to shed some understanding on the role of genetic aberrations in the success rates of various myeloma therapies, adding still more complexity and uncertainty to the treatment decision-making (TDM) process.18, 19 The advent of novel drugs showing similar (or sometimes better) response rates when compared historically to the outcomes from standard therapies creates further clinical uncertainties in treatment decision making.20-23 Research studies that examine not only the physician’s perspectives, but also those of the patient, can inform both clinicians and policy makers on how to improve outcomes related to cancer treatment decisions. By examining and understanding patient preferences and values, clinicians will be better prepared to engage in shared decision making with patients diagnosed with myeloma. Information on treatment decision making is particularly relevant for the elderly with myeloma, who may have a different set of values and preferences than younger patients. Conversely, by understanding physician perspectives, policy makers and medical practice administrators will have a broader view of the process and may be able to support innovative strategies that will enhance physician-patient treatment decision encounters. Treatment Considerations in Older Adults There are specific treatment considerations in older adults with cancer, particularly those who are 60 years of age or older. The patient’s age is likely to be an influential factor in 58 treatment decision making by both patients and clinicians. Berry and colleagues24 found evidence of this association in a study of 260 men with localized prostate cancer. A majority (70%) of the study participants reported that their age had influenced their treatment decision, with older men being more likely to eliminate a particular treatment option exclusively because of their advanced age. In addition, several studies have found that clinicians will either rule out particular treatments for patients with colorectal or breast cancer based on a patient’s age or will give strong recommendations against particular treatments.25-29 In a recent survey30 of physicians who were involved in treatment decisions regarding chemotherapy in cancer patients ages 70 years and older, treatment side effects (24.4%), multiple illnesses (20.5%), and lack of support from family and friends (10.9%) were reported as challenges. The authors reported that in addition to the presence of comorbidities, functional status was among the principal factors physicians considered when they made such treatment decisions.30 Older patients are at a higher risk for chemotherapy toxicities due to physiological changes associated with aging, potentially causing adverse quality of life (QOL) outcomes.31 QOL, comorbidities, treatment tolerance, and life expectancy have been proposed as important elements in the treatment decision equation of older adults with cancer.32-34 In fact, older adults have ranked QOL as a top priority in life.35 When asked about the importance between QOL and quantity of life in relation to their treatment decisions, 97% (N=42) of older adults (age range 60-85 years, median 71 years) with acute myeloid leukemia or advanced myelodysplastic syndrome shared that QOL, rather than length of life, was an important factor in their choice of therapy.36 59 The elderly population is growing rapidly and life expectancy is rising; therefore, studies that examine the impact of QOL, comorbidities, and life expectancy on treatment selection are increasingly becoming critical. How these various factors are considered in the context of patient-physician interactions during treatment decision making has not been explored in the past. In summary, there is strong evidence that older patients with cancer do have personal preferences and values and contextual factors influencing their treatment decisions. There is also considerable evidence that patients want to be informed and consulted with regard to the impact of treatment on quality of life as well as survival. However, no data exist regarding influential factors that older adults diagnosed with myeloma consider when they make treatment decisions. Moreover, physician factors influencing treatment decisions have not been previously studied in older adults newly diagnosed with myeloma. Purpose The purpose of this study was to explore patient- and physician-related factors influencing treatment decisions in older adults newly diagnosed with myeloma. The first objective of the study was to examine the patients’ perspectives on treatment decision making, including their personal and contextual factors relevant to treatment decision making. The second objective was to describe physicians’ perspectives on the treatment decision making in older adults (≥60 years of age) newly diagnosed with myeloma. Methods Design The study employed a descriptive, cross-sectional design using semi-structured interviews. Since treatment decision making is a complex health care phenomenon that has 60 not been elaborated in patients with multiple myeloma, a qualitative approach was used to explore the perspectives of patients and physicians during treatment decision making and to examine the factors influencing treatment decisions from both perspectives. Sample The patient sample consisted of 20 older adults ages 60 years and above who were newly diagnosed with myeloma and had been referred to Seattle Cancer Care Alliance (SCCA) or the Northwestern University Myeloma Program (NUMP) by hematologists/oncologists in the greater Seattle or Chicago areas, respectively. To be eligible for the study, patients had to be (a) older adults (60 years of age and above); (b) newly diagnosed (6 months from diagnosis) with multiple myeloma; (c) able to read and write English; (d) able and willing to give informed consent. The physician sample consisted of five physicians from SCCA and University of Washington (UW)-affiliated clinics and five physicians from NUMP who were directly providing care to myeloma patients. Patient and Physician Recruitment After obtaining approval from the UW and Northwestern University Human Subjects Divisions (HSD), older adults newly diagnosed with myeloma were recruited to participate in the study. The researcher made every attempt to recruit from both university- and community-based practices to enhance the diversity of study participants. Older adults ages 60 and older, who had an appointment at either SCCA’s, UW-affiliated clinics’, or NUMP’s hematology or transplant services and were otherwise eligible for the study based on clinic records were recruited by mail using a recruitment flyer (SCCA or UW clinics) or a direct approach (NUMP). Clinic schedules were reviewed weekly to identify potential study participants. A total of 79 potential participants at SCCA and UW-affiliated clinics were sent 61 recruitment letters from October 2009 through July 2010. Of these 79 potential participants, 14 responded to the mailer (17.7 % response rate), and all agreed to participate in the study. At NUMP all six potential participants approached by the researcher agreed to participate. Physicians were recruited via e-mail and by direct approach. A total of 16 physicians were approached from SCCA, UW-affiliated community clinics, and NUMP. Three physicians from SCCA, two physicians from UW-affiliated clinics and five physicians from NUMP agreed to participate (62.5% recruitment rate). Informed consent was obtained from all study participants. No enrolled participants withdrew from the study before complete data were collected. Procedure A semi-structured interview was conducted in a designated research-related conference room at SCCA and NUMP. These rooms were strictly assigned for research use only and met the HSD’s standard for patient privacy. If a patient wanted the interview to be conducted at a later time, a one-week period following the clinic appointment was allowed for re-scheduling. Moreover, if a patient requested, the researcher conducted the interview at his or her home. Similarly, the physician interview was conducted in a place where privacy was secured, generally the physician’s office. Patient participants were asked about the treatment options discussed by their physicians including risks and benefits, their preferred role during decision making, and how they make the best treatment decisions for themselves. Physician participants were asked to recall their experiences of how they usually presented treatment options to patients. They were then asked specifically about which factors they consider when making a treatment 62 decision, their preferences and perceptions of patient participation during the decisionmaking process, and how they make the best treatment decision for their patients. All study interviews were audio-recorded and then transcribed verbatim. Identifying names or proper nouns were not included in the transcription. All transcripts were checked against the original audio recording for accuracy. Analysis Directed content analysis procedures37 were used to develop major themes from the patient and physician participant interviews. Initial categories and their definitions were developed based on a literature review of physician and patient factors influencing treatment decisions in cancer. Interview text was read line by line by the lead investigator (JDT) and then imported to NVivo 8 (QSR International, Victoria, Australia),38 a qualitative data analytic software program. Initial categories and definitions were also imported to NVivo. The minimum unit of analysis was typically one sentence, but sometimes the unit was an entire paragraph, depending on whether the participant shifted the topic in a different direction other than what was asked in the interview schedule or in follow-up probes. Probes included statements such as, “Tell me more about the role you have selected,” or “What else are the influential factors in your treatment decisions?” If the interview text matched the definition of a pre-established category, that code was assigned to the text. Text that could not be coded within the initial categories was given a new category and definition. Some categories were grouped together to create major themes. Interview transcripts were re-coded based on the subsequent identification and definition of these new themes or other new categories. 63 Full agreement between investigator JD and BC in terms of coding scheme and their definitions was reached utilizing the process of consensual validation.39 Initial and emerging categories were reviewed and discussed among three members of the research team (JD, BC, and DLB). Ongoing in-depth discussions and agreement about the wording of final themes, factors encompassed by major themes, and their definitions were carried out by the investigators, JD and BC. The percentage agreement for the coding of patient interview themes and factors was 86.7% and 81.4%, respectively. For physician interview themes and factors, the percentage agreement was 91.7% and 86.1%, respectively. Given the exploratory nature of this study, the degree of agreement for coding that has been achieved between investigator JD and BC is considered acceptable.40 Nine major themes were identified from the patient interviews and seven major themes were identified from the physician interviews. Results The patient participants sample mean age was 67.5 years (SD=7.6); mostly Caucasian men and women participated, with only one Asian and one Native American. The sample of ten physician participants consisted mainly of women (n=7) between 30 and 39 years of age (n=8), but with varying race/ethnicity and title or position in the institution. Tables 1 and 2 list all demographic information collected for the patient and physician participants, respectively. 64 Table 1. Sociodemographic Characteristics of the Patient Sample Variable Age group (mean 67.5 years) 60-70 71-82 Gender Male Female Race Caucasian Asian American Indian/Alaskan Native Employment Status Full time Working, on medical leave Not working Retired Student Personal Relationship Status Single Married or partnered Divorced Widowed Highest Level of Education 9th – 12th grade 2 years of college 4 years of college Graduate degree Annual Household Income $18,000 or less $18,000 to $35,000 $35,001 to $55,000 $55,001 to $85,000 $85,001 and above N % 14 6 70 30 8 12 40 60 18 1 1 90 5 5 2 2 2 13 1 10 10 10 65 5 2 12 5 1 10 60 25 5 5 2 10 3 25 10 50 15 3 2 5 5 5 15 10 25 25 25 65 Table 2. Sociodemographic Characteristics of the Physician Sample Variable Age group (mean??) 30-39 40-59 Gender Male Female Race Caucasian Asian African American Title or Position Fellow Attending Physician Private Practice Physician Personal Relationship Status Single Married or partnered N % 8 2 80 20 3 7 30 70 5 3 2 50 30 20 5 3 2 50 30 20 1 9 10 90 The major themes with their definitions and frequencies of occurrences for both patient and physician interviews are shown in Tables 3 and 4. Table 3. Major Themes from Patient Participant Interviews on TDM Themes Trust in the physician, health care team, and/or institution Definitions Participants verbally expressed their trust in the physician, the health care team, and/or the institution as influential in treatment decisions. This definition also includes implicit trust to the physician by going along with physician’s treatment recommendations or decisions. N (%) 20 (100) 66 Table 3 continued Themes Participants have many sources of information related to myeloma Participants have various decisional role preferences Patient-specific factors influence treatment decisions Negative perceptions of treatment decision making Definitions Participants described the different sources of myeloma-related information such the Internet, physicians, family and friends who help do the research and obtain myeloma-related materials, physician visit companions, books, pamphlets, nurses, myeloma patients, other cancer patients, and support group such as the multiple myeloma fighters and myeloma or lymphoma society. This is distinct from “other’s opinions” in which the information or opinion was identified as actually influencing the treatment decision Patients described their role preferences or any changes in role preferences as being active (patient making his or own treatment decision with or without consideration of the physician’s opinion), shared (patient and physician share responsibilities in making the treatment decision), or passive (patient delegating the treatment decision to the physician) or changes in their role preferences outside of the context of being in a state of shock. Patient-specific factors refer to patient’s actual experience with myeloma-related therapy, age, beliefs and values, faith in a higher power, opinions of family, opinions of others, past health-related experience not related to myeloma, and self description of “What I’m like” influencing treatment decisions. This theme will be coded along with the patient-specific factor. Patients described negative perceptions of treatment decision-making such as lack of discussion of treatment options, long periods of waiting during the encounter, inability to reach a health care team member, and wanting to have more information related to disease, prognosis, treatment, and side effects or having questions left unanswered, not achieving the desired level of participation N (%) 20 (100) 20 (100) 19 (95) 17 (85) 67 Table 3 continued Themes Treatment decisions are driven by the benefits of being cancer-free, the desire to be in remission, and the desire to live a longer life Contextual factors influence treatment decisions Some participants were in a state of shock at the time of diagnosis Advances in science provide hope for future treatment options Definitions Patients described the benefits of their therapy such as being cancer-free, killing the cancer cells (patients also describe their myeloma marker at 0 level), being in remission, and living a long life Patients’ contextual factors refer to issues of health insurance, financial status, availability of free medication regardless of insurance, geographical barriers, treatment costs, social support, housing/lodging, retirement planning, recent significant events in the family, and transportation/convenience of oral therapy at home influencing treatment decisions. This theme will be coded along with the specific contextual factor. Participants described being in a state of shock, feeling very overwhelmed and not at the right frame of mind, unable to process what was heard from the physicians during the visit, feeling pretty much out of it or kind of in a fog, and feeling paralyzed from participating in treatment decision making Participants described advances in science provide hope for future treatment options but not influencing their current treatment decision N (%) 15 (75) 14 (70) 6 (30) 4 (20) 68 Table 4. Major Themes from Physician Participant Interviews on TDM Themes Definitions Some physicians describe QOL or survival consideration and some physicians simultaneously consider multiple factors including efficacy, QOL, and survival in their treatment decisions. This theme is also coded when the physician mentions morbidity, mortality, and life expectancy considerations when making treatment decisions. These are aspects of the physician’s life that Physician-specific influence treatment decisions. These factors factors influence include patient’s context, patient’s family treatment decisions opinion, patient’s co-morbidities, functional status, and supportive care requirement, patient’s treatment preference, patient’s age, patient’s medical and clinical factors, physician’s beliefs and values, and physician’s expertise and type of practice. This theme is coded in conjunction with the specific physician factors. Physicians evaluate their patients’ overall medical Eligibility for autologous HSCT is an condition for eligibility for autologous stem cell transplantation important treatment consideration Physicians use various Physicians share that they have no systematic tool to assess preference; sometimes they ask or ways of assessing patient decisional role sometimes they indicate they just have a feeling for the patient’s role preference. This theme does preferences not include the physician’s own description of the decisional role of patients. Physicians describe limited time and lack of long Barriers to effective term outcome data as barriers to effective decision making decision making; physicians share that there is a need to spend more time talking with patient The patient ultimately Physicians describe providing different treatment options to patients, explaining risk and benefits, makes the treatment and specifically state that patient ultimately decision makes the final decision. This does not include physician’s belief on patient participation or nonparticipation with decision making Physicians consider QOL or survival alone or simultaneously consider QOL, treatment effectiveness, and survival N (%) 10 (100) 10 (100) 9 (90) 9 (90) 7 (70) 5 (50) 69 Table 4 continued Themes Definitions N (%) 2 (20) Physicians describe presenting strong treatment When needed, recommendations to patients when patients make physicians attempt to illogical decisions persuade patients to take the physician’s recommended treatment option Abbreviations: QOL = quality of life; TDM = treatment decision making; HSCT = hematopoietic stem cell transplantation Major Themes from Patient Interviews The major themes from the patient participant interviews were organized according to the highest to lowest frequency of coding and are presented below. Trust in the Physician, the Health Care Team and the Institution All participants considered the physician’s opinion as having an influence on their treatment decisions. Twelve out of twenty participants explicitly said they trusted their physicians immediately at the time of diagnosis and accordingly went along with the physician’s treatment decision. Eight participants indicated implicit trust of their physician’s treatment decision by going along with the physician or not questioning the physician’s treatment decisions. Additionally, in cases where a physician had expressed a preference for a particular treatment, no patient participants described going against the physician’s preferred therapy. Finally, based on the physician’s evaluation of their myeloma status, patient participants tended to agree with the next treatment direction the physician suggested. Regardless of the physician practice type or specialty, there was a consistent theme of trust in the physician. One patient participant explicitly mentioned trust in the physician’s team and the institution (NUMP). 70 Patient 005: “I really want to trust his expertise because he knows a lot more about it than I do, so he probably has more input than I have.” Decisional Role Preferences Participants expressed various decisional role preferences. Half of the participants preferred to make the decision themselves, while 45% said they would have liked to share the responsibility of making the decision with their physician. Only one patient participant brought up the idea that she would have liked the physician to make the decision while seriously considering her opinions about treatment before making a recommendation. Sources of Information Related to Myeloma When patient participants were asked where they get their information related to myeloma and its therapy, various sources, including internet (n=12); physicians, both primary oncologist and second opinion physicians (n=7); family and friends (n=4); books (n=3); pamphlets (n=3); nurses (n=2); myeloma patients or other cancer patients (n=3). Some patients reported two or three different sources of information at any given time. Contextual and Patient-specific Factors Influence Treatment Decisions When asked how they make the “best treatment decision,” participants described larger contexts in their lives, talking about factors such as finances (impact on work, retirement), logistics (transportation, housing/lodging), availability of family/caregiver support, existence of an elderly spouse or another sick person of the family, living status (living alone), and geographical advantage (living near a transplant center) as being influential in their own treatment selection. An exemplar quote from a patient who decided to have intravenous bortezomib therapy instead of oral chemotherapy: Patient 013: “I have someone to drive me to the clinic; my wife does that. I drive over and she drives back. I feel capable of driving back, but my concern is that if I’m in an 71 accident and they learn that I just came from a clinic, something will be wrong with my responses, so we don’t take that chance. I just let her drive me back and that works fine.” Aside from contextual factors, there are several patient-specific factors that they mentioned as influential in their treatment choice. These factors pertain to the individual’s recall of influences on their actual treatment selection. Tables 5 and 6 outline these patientspecific and contextual factors, respectively. The most common patient-specific factors influencing treatment decisions were actual experience and the value placed on quality of life. Patient participants described how their actual experience with therapy influenced their treatment decisions. They were able to recognize that, when they are not responding to therapy, a change of therapy is most likely going to happen. Additionally, when they are experiencing unacceptable toxicity from the therapy, it also meant modification in their treatment plan or change of therapy. The patient participants wanted to live a physically active and productive life, continuing to do things such as driving, skiing, traveling, and spending time with family and friends. They considered the most appealing therapies to be those that would not make them feel sick all the time with nausea, vomiting or pain. They preferred not to have blood transfusions several times a week. Participants also said that, if it was possible, they did not want to visit the clinic/physician several times a month. Oral chemotherapy was preferred by six patient participants who were above the age of 70 years. They did not want to continue therapy if it meant not being able to do the things they loved. A patient participant said, “If I needed to spend 8 years in a wheelchair, with no one to look after me, by myself somewhere, not being able to paint, not being able to write, I don’t see the point of it.” 72 Table 5. Patient-specific Factors that Influenced Treatment Decisions Factors Actual experience with therapy Beliefs and values Opinions of family Definitions Participant’s actual experience with therapy specific for their myeloma such as reaction, side effects, response or nonresponse to therapy influence subsequent decisions. This definition does not include experiences with therapies not related to myeloma (included in the definition of past healthrelated experiences) Participants’ personal belief about the necessity of completing a therapy or belief in the outcomes of a specific therapy and the participants’ valuation of QOL, independence, and not being a burden to family as influences on their treatment decisions. Beliefs and values are different from the perceived benefits of treatment Participants’ solicitation and consideration of the opinions of family influenced treatment decisions; a specific link between family opinions and treatment choice was identified Exemplar Quotes “I had unfavorable reactions to the medication I was taking. I started developing neuropathy in my hands and feet. My doctor consulted a myeloma specialist and my treatment was changed.” N (%) 16 (80) “I just think this cancer is very tricky and that we have to outtrick it. So I think novel treatments are where I see the greatest potential of a longer life for people like me. That’s why I chose the clinical trial involving novel agents.” 16 (80) “My children very much wanted me to fight this to the bitter end and regain my life back, because I think it was very hard for them to see me in an invalid kind of stage where they had to care for me at the beginning, and I’m always the one caring for them.” 9 (45) 73 Table 5 continued Factors Age Definitions Participants who described themselves as being at a particular age category and feeling healthy or being in a particular age, regardless of health status, had influenced their treatment decision Participant’s solicitation and consideration of the opinions of non-family members influenced treatment decision; a specific link between opinions of others and treatment choice was identified Exemplar Quotes “Well, the option that was not seriously considered was stem cell transplantation; because of my age [80 years] that was ruled out.” “The thing that did influence me a lot was I talked to a gentleman during chemo that had gone through the stem cell and he was telling me how he had eight absolutely wonderful years where he traveled and he was free of cancer and he got his life back.” "I had 21 operations in my life. Past health- Participant’s own past healthrelated (e.g., overall good I’ve come through all that. related health, past illness experience) This is just another step in my experience and therapy-related life and I'll come out the other experiences (not relating to end smiling. So that is why I myeloma) influenced decided to take the decision of participants’ treatment choice having an auto stem cell transplant.” “I didn't see any reason to “What I’m Participants who identified what they were like as a question there was an like” person—their job, their alternative to this cutting edge personality--influenced treatment [auto stem cell treatment decisions transplant]. It seemed to me I'm a cutting edge guy so it appealed to me.” Participants described praying “I prayed to God to give me Faith in a to a higher power and faith in a the best treatment and the best higher higher power as an influence in doctor.” power their treatment decision Opinions of others N (%) 9 (45) 8 (40) 6 (30) 5 (25) 5 (25) 74 Table 6. Contextual Factors that Influence Treatment Decisions Factors Definitions Social support Availability of family and/or friends to provide caregiver support during therapy-including attendance at the physician or clinic visits or availability of family members to take some household responsibilities, being single, family, and caregiver burden-influenced treatment decision. The type of insurance coverage for a particular therapy influenced participants’ treatment choice. Insurance Transportation issues/ Convenience of pills Geographic barrier Exemplar Quotes “My wife is an absolute jewel. She insists on taking care of my every need and she invents some needs I probably don’t think about. My concern is if she will wear down. She’s 83 and she has a lot of energy and she’s a wonderful caregiver, but I worry that I may wear her down.” “We were glad that we have very good insurance coverage. For two transplants, I was adamant that any procedure I do, it has to be certified and pre-approved [by the insurance company].” Travel issues from home to “Basically my doctor offered clinic to home--such as either the IV or the Revlimid availability of a driver or the by pill. I felt that well, I guess ability of the participant to the pill was more appropriate drive a car, or the convenience for quality of life from my of taking oral chemotherapy at standpoint. It's easier to do, it home--influenced treatment gives me more flexibility, and decisions. I don't have to keep going in to the clinic every day.” Participants described the “I considered Mayo. I have a actual distance and amount of relationship with them from time to travel to get the therapy many years ago, but I wanted influenced treatment decision to be in town [Chicago]. I'm and the need for housing or impressed with the team in lodging during therapy due to Northwestern facility and it distance of medical institution also has the advantages of it for the participant’s residence. being near my three daughters and my wife so that I didn't see any reason to go anywhere else.” N (%) 9 (45) 5 (25) 5 (25) 5 (25) 75 Table 6 continued Factors Definitions Finances The participant verbally expressed that the amount of money the participant has to pay out of his own pocket to get the therapy and costs of retirement influenced treatment decision. This factor includes participants getting free medication instead of paying several thousands of dollars for their chemotherapy Participants described how significant events or conditions in the family such as another sick member of the family or recent family death influenced treatment decision. Significant events in the family Exemplar Quotes “I'm running on empty [finances] now. So I do take that into account.” “My mother is ailing and she's very old, and we don't know what our family needs are in taking care of her needs. But I have wanted to avoid having a transplant, if possible.” N (%) 5 (25) 3 (15) Negative Perceptions of the Treatment Decision-making (TDM) Process The majority of the patient participants shared negative aspects of TDM. The most common negative observations they made were their physicians being too busy and lacking the time to adequately discuss treatment options or side effects of therapy. Fourteen percent of the participants thought the physician did not present treatment options and that the side effects of therapy were not discussed adequately. One participant thought her concerns were not addressed at all. Patient 008: “The first time we saw [the doctor], we waited and waited and waited and waited and thought, "Oh my gosh, maybe we should just leave." So she's just a very busy person.” More than half of the participants expressed that some aspects of their information needs were not met by their physicians. These needs related to the current and subsequent treatments; overall survival; future therapies; diagnostic and monitoring tests; side effects 76 and their management and just a general overview of myeloma, including its causes and genetic impact on the family. Patient 001: “I think [information about] the treatment, length of treatment was very iffy—it was kind of like: Let’s see what happens. And possible side effects were not really discussed.” Treatment decisions are driven by the benefits of being cancer-free, the desire to be in remission, and the desire to live a longer life When patient participants were asked to share their understanding of the benefits of the treatment they currently have, the majority (75%) stated that they chose the treatment they had because it was presented to them by the physician as their best chance to be cancerfree, to be in remission, and to get them back again to living a longer life. The patient participants were cognizant that currently there is no cure for myeloma and that their treatments offer them a period of remission, but relapses are inevitable. Patient 001: “My doctor was very positive about the effects of the stem cell transplant being the only way to achieve any kind … [pause] … Well, I was told this is a non-curable disease, which I know. He told me the benefits of a longer remission, putting me to a more normal life expectancy.” State of Shock at the Time of Diagnosis Six of the participants described themselves as being in shock when given the diagnosis of myeloma. They described their reactions as “pretty much being out of it” and “kind of in a fog” when told about the myeloma diagnosis. One participant said, “This is just almost too much to take and I didn’t want to live anymore.” For these participants, it was just too much to cope with the diagnosis and they were unable to participate in the decisionmaking process. 77 Patient 007: “I was kind of in a fog when it hit me. I was just kind of … I brought my wife so she could hear what was being said and I’m glad I did, because a lot of things were being explained that I didn’t pick up on because of the—I guess for lack of a better word— the shock of being diagnosed with cancer.” Some patient participants did not require immediate treatment decisions at the time of diagnosis. They were able to think about their options for a week or two and had opportunities to discuss the different treatment options with their families and friends; some had the time to seek a second opinion with another physician. Hope for Advances in Science Participants shared their perspectives on how they rely on science to help them have more treatment options. They have a good understanding that the science of myeloma therapy is constantly evolving and that there have been several new drugs approved as treatments for myeloma. However, these patient participants did not make their decisions based on the hope for advances in science. They made their decisions based on what treatment options were currently available to them, with the hope that advances in science will be available to them in the near future. Patient 010: “The only wildcard in this whole medical history is that because myeloma is a cellular disease involving a lot of genetic components, and that part of our science is evolving very quickly, I assume that if I can keep myself alive for a few years that the medical technology may change.” Major Themes for Physician Interviews Physicians Consider QOL or Survival Alone or Simultaneously Consider Treatment Effectiveness, QOL and Survival According to the physician participants, there were three main components of a treatment decision: balancing of treatment success (i.e., achieving remission), QOL, and overall survival or considering each component separately. One physician participant 78 recognized that some patients would want as much quantity of life as possible in the beginning, but as patients realized that QOL might decline with aggressive treatment, they would backpedal and re-balance their treatment goals, with QOL being given relatively more weight. The majority of physician participants agreed that for the elderly patients, QOL is more important than quantity of life; only one physician participant discussed remission and survival without explicitly talking about QOL. Physician 009: “I think most patients can agree that a minimal decrease in the quality of life over a short term in exchange for potentially a positive outcome in the long term is worth it. But again, you have to take that into effect of how long are we affecting the patient’s quality of life? You don’t want to keep somebody alive, you know—we can keep somebody alive nowadays as long as we want sometimes, you know, and you don’t want to do that in the setting of a very poor quality of life.” In order to strike a balance among treatment success, QOL, and survival, physician participants described the many treatment decision factors that they consider when recommending treatment options to patients. Additionally, when asked how they make the “best decision” for their patients, the physician participants identified many factors that they consider during decision making. Table 7 outlines the factors that physician participants reported as influential in the treatment decision. Table 7. Physician-specific Factors Influencing Treatment Decisions Factors Patient’s co-morbidities, functional status, and supportive care requirements Definitions Exemplar Quotes Physicians describe presence of comorbidities, poor or good functional status, and supportive care issues influencing choice of chemotherapy approach “Other medical conditions, performance status, if they've had complications with the chemo’ before. Primarily comorbid conditions and their performance status would make me choose a less intensive approach.” N (%) 10 (100) 79 Table 7 continued Factors Physician’s beliefs and values Patient’s context Definitions Exemplar Quotes Physician’s personal beliefs on patient-physician relationship dynamics; beliefs that the patient does not have much knowledge, need for oversimplification, need for slowly introducing myeloma concepts, and patient asking the physician to talk about what treatment physicians would choose if it were them; beliefs that patients should or should not participate in decision making; belief that myeloma decisions are becoming more technically difficult to understand for the patient influence treatment decisions Physicians consider the patients’ contextual factors such as health insurance, financial status, availability of free medication regardless of insurance, geographical barriers, treatment costs, social support, housing/lodging issue, transportation/convenience of oral therapy at home, and treatment compliance issues as influential factors in treatment “I think that would still involve me being very active—because I believe in transplantation I think that I would be—try to be very convincing to—that would be wise. Because that’s the approach I believe in.” “The patient’s overall situation could alter my decision; their ability to come to the doctor, their ability to follow-up, could decide whether I would pick a non-transplant or transplant approach.” N (%) 10 (100) 9 (90) 80 Table 7 continued Factors Definitions Exemplar Quotes Patient’s medical and clinical factors Physicians consider patientspecific medical and clinical factors such as type of myeloma, high-risk disease features by cytogenetics, fluorescent in situ hybridization (FISH) test, or genomics, positive response or resistance to therapy, patient’s actual experience with therapy, and any end-organ damage as influential factors in treatment selection Physician’s expertise and type of practice The physician’s expertise in stem cell transplantation and practice type (transplant center) influence treatment choice Patient’s age Consideration of patient’s age (at 70 or older) regardless of other factors, influenced choice of a non-transplant option or very young myeloma patients ages 40-50 years old tending to have very aggressive treatment such as high dose chemotherapy. Agerelated issues in the context of stem cell transplant discussion was coded under eligibility for HSCT “The prognostic indicators of the cytogenetics of the bone marrow will also lead a little bit your decision making, and the treatment of multiple myeloma in the sense that if you have deletion 17, we know that myeloma won’t be responsive to thalidomide or lenalidomide or the “-imides” in general. Therefore, you would start an induction treatment with bortezomib typically.” “I think to some degree it’s our own bias because we are a transplant center and I and most of my colleagues continue to believe that for the appropriate age and health condition, transplant is of value. And so I definitely approach a new patient with the idea of determining if they’re a suitable patient for transplant.” “I choose certain drugs for patients who are age 70 and over; certainly over this age we would consider non-transplant drugs.” N (%) 7 (70) 6 (60) 6 (60) 81 Table 7 continued Factors Patient’s treatment preference Family opinion Clinical trials as an option Definitions Exemplar Quotes Patient’s expressed preference for a specific therapy influenced treatment choice “If they [patients] say they just want pills and they understand all of the upsides and downsides, then sure.” Family member’s opinion “The older the patient the more being weighed in by likely you are to have other physicians as an influence in family members involved. their treatment choice, but does More than just the spouse. It not include physician’s might be the daughter, or the mention of the presence of a kids involved. It is important family member during patient- to get to the point where physician encounters everyone is comfortable and understands what, and is in agreement with the goals of treatment.” Offering a clinical trial as a “For the patients over age 70 treatment option regardless of or certainly over age 75, we’d availability of free medication either be considering our influenced treatment decisions clinical trial, which is lenalidomide/dexamethasone until progression, lenalidomide/dexamethasone for 18 months, or melphalan/prednisone/thalidomide.” N (%) 5 (50) 3 (30) 2 (20) Eligibility for Autologous HSCT When physician participants were asked about the treatment options available to older adults newly diagnosed with myeloma, the majority (90%) said they check for patient’s eligibility for autologous hematopoietic stem cell transplantation (HSCT). There were at least four physician participants who considered the cut-off age for HSCT as being 70. Two physician participants said they would still consider HSCT after the age of 70, but only if their patients did not have comorbidities and had an excellent performance status. Only one physician participant did not talk about HSCT and talked instead about non-transplant 82 treatment options, which is possibly due to the advanced age (over 70 years of age) of myeloma patients under his care. Physician 004: “Usually you break down the treatment options into considering if the patient is a transplant candidate or not--if they’re a candidate for autologous stem cell transplant. So usually, the patients who are 60 to 70 we might consider in that category.” Barriers to Effective TDM Some physician participants felt the time allotted for the clinic encounter is not enough for adequate discussion of treatment options. One physician participant also thought that explaining alternative treatment options to patients and allowing patients to express their treatment preference are aspects of TDM that need some improvement. The physician participants recognized that time management is an important aspect of patient care. Physician 001: “Time is a major barrier, yeah. If you have to say… Listen, you have to do everything in a half an hour—not possible—or even sometimes… You know, new cases that come are usually an hour, an hour and a half with the attending … just one hour if you’re just an attending sometimes is really not enough.” Physician 007: “It's not really realistic. Especially if you have a lot of patients that you're seeing in the clinic in a day. Then you might not have one hour to sit down with every single patient and go over every single detail of the patient's treatment. So in an ideal world, you spend one hour with every single patient, trying to explain every single detail. But sometimes I think, because we have a lot of patients to look after, we basically are more— our discussions with patients might be brief.” Physicians Use Various Ways of Assessing Patient Decisional Role Preferences When physician participants were asked whether they had a systematic way of assessing a patient’s decisional role preference (e.g., use a tool to elicit patient’s decisional role preference), nine out of ten responded that they do not have a systematic approach. The physician participants commonly used various communication methods and observational skills to gauge the decisional role the patient prefers during treatment decision making. Most 83 of the physician participants said they can tell what decisional role the patients might prefer based on the kinds of questions they ask and sometimes by asking them directly. Physician 003: “I don’t have a systematic way to do it, but usually by interviewing them and meeting with them and talking to them, I think you can get a better sense of the degree to which they want to be active participants in how they’re treated.” The Patient Ultimately Makes the Final Treatment Decision The majority of the physician participants (80%) described their role during treatment decision making as being that of someone who presents the options to the patients and gives them the opportunity to make the treatment decision. Most physician participants said they tried to educate their patients, listen to them, and provide them with options that meet patient needs or expectations. Two physician participants explicitly expressed that they will respect the patient’s preferences and choices as long as effectiveness of therapy and overall outcome were not compromised. Physician 001: “In the end, it’s the patient that decides what he or she wants to do. And so, what I can do is I can say to him, “There is this treatment, that treatment, these are the risks involved with this treatment or that treatment, and these are the benefits. But ultimately, it’s the patient who decides what he or she wants to do.” When Needed, Physicians Attempt to Persuade Patients to Take Their Recommended Treatment Option Two physician participants stated that when patients do not want to do anything or want to do something outside of their recommendations, they try to persuade patients to take their recommendations. Additionally, when presenting options to patients, the physician participants try to explain to their patients why they would favor one treatment option over the others. The physician participants were cognizant that they cannot force patients to do what they do not want to do, and physician participants emphasize that treatment decision making is a joint process between the patient and the physician. 84 Physician 001: “Let’s say that if somebody comes with stage III high-risk multiple myeloma and says, “We don’t want to do anything,” then we will strongly recommend—we will strongly recommend doing something.” Discussion These findings document many themes and factors considered by physicians and older patients with multiple myeloma during treatment decision making. There are some similarities between the physician and patient results regarding influential factors for treatment selection such as quality of life, convenience, insurance, cost, family opinion, age, patient’s medical and clinical factors and social support considerations. These multidimensional factors are simultaneously weighed by patients and physicians to make the “best decision” in the setting of clinical uncertainty. Berry and colleagues41 have previously reported similar personal factors that were influential in the treatment choices of men diagnosed with localized prostate cancer, including age, cancer in the family, family responsibilities and desire for longevity, as well as physician factors that included consideration of the patient’s comorbidity and pathology. Maintaining quality of life during therapy is very important, not only from the patient participant’s perspective but also from the physician participant’s point of view. Among the contextual factors described by patient participants, the convenience of oral chemotherapy has been described as influential in the treatment decisions. Since a pill can be conveniently taken at home, a decision for oral chemotherapy translates to fewer visits to the clinic, ultimately impacting patients’ overall quality of life. This option is very attractive to older adults above the age of 70 years participating in this study, because it offers them more independence and requires less family burden to complete the therapy when they do not need to ask family members to drive them to the clinic. 85 QOL and independence are values that are consistently ranked by older adults as their top priorities in life.35, 36 Husain et al.42 have also reported quality of life and independence as influential factors in the treatment choices by older women (>70 years of age) with breast cancer. For physician participants in this study, oral chemotherapy was appealing as long as the patients could adhere to the prescribed therapy and treatment efficacy was not compromised. Kreling and colleagues43 have reported some similarities in what they called patient’s contextual factors influencing treatment decision in older women (≥65 years of age) with breast cancer, including the patient’s age, functional status, comorbidities and perceptions of the benefits and side effects of chemotherapy. The patient participants were making treatment choices based on contexts in their lives. For example, the availability of support from family and friends influenced the patients’ choice of intensive therapy. Intensive therapy requires a significant time commitment and caregiver support during the acute phase of therapy (typically, days 1-30 post-transplant). The physician participants were cognizant of patient preferences and contextual factors and offered treatment recommendations with strong consideration of the patients’ overall personal factors and social contexts. The physician participants talked about the importance of assessing a patient’s financial, logistical, and social support status and how these factors influenced their treatment recommendations to their patients. One study44 has reported family burden, cost, and travel requirements as important factors influencing the physician’s decision to use adjuvant chemotherapy among older adults (≥65 years of age) diagnosed with stage III colon cancer, but these contextual factors were not ranked as important as patients’ comorbidities and medical evidence for treatment. There are some similarities in the patient-specific factors (i.e., age, past health-related experience, insurance, 86 social support, family burden, geographic barrier) influencing treatment decisions when compared to patient-specific factors of other older patients diagnosed with cancer. However, some of the personal and social contexts (i.e., actual experience, some aspects of personal beliefs and values, opinion of others, significant events in the family, convenience of oral pills, and faith in high power) in this study varied from the personal and social contexts in patients with breast,42 prostate,13 ovarian cancer,43 and colorectal cancer.44 At a certain point, age becomes influential in making a treatment choice; in general, this study revealed that physician participants consider 70 as the cut-off age for more intensive chemotherapies. In this study, patient participants also considered their particular age as influential in their treatment choice. For example, patient participants who were 70 years and above (n=6) preferred oral chemotherapy and did not consider intensive chemotherapy. The older patient’s preference for intensive or non-intensive therapy could have been influenced by their physician’s recommendation, as this study has documented. Kutner and colleagues44 have reported similar patient-reported considerations of the physician’s opinion as influential in treatment decisions. Notwithstanding their findings, some physician participants in this study would still consider intensive chemotherapy for patients above the age of 70, provided that the patient had good performance status and no comorbidities. Physician participants strongly considered treatment effectiveness and overall survival, albeit while keeping an eye on the patient’s quality of life. The patient’s age has been reported by both patient and physician as an influential factor in their treatment decisions in patients with breast42 and prostate cancers.41 It was not surprising that both the patient (≤65 years of age) and physician participants were considering intensive therapy. Intensive chemotherapy using high-dose 87 melphalan followed by autologous HSCT is considered an important treatment option for myeloma patients under the age of 65.45, 46 For patients above the age of 65, it is unclear whether physicians use a standardized decision analysis tool in their treatment recommendations for intensive versus non-intensive therapy. Some physicians would not recommend intensive therapy after the age of 70 years; others would still consider intensive therapy beyond the age of 70, depending on the patient’s functional status and overall health condition. The decision dilemma to recommend or not recommend intensive therapy to older adults with myeloma has been raised in a review paper, where the authors of the paper asked whether physicians are screening patients appropriately for intensive therapy.47 More studies are needed to develop innovative methods to help both patients and physicians reach a consensus on which treatment approach (e.g., intensive versus non-intensive) is best to take in a given context. Furthermore, more studies are needed to help physicians develop clinical decision pathways or treatment decision algorithms with regard to screening patients for eligibility for autologous stem cell transplantation. There was evidence of discordance between the physician’s and patient’s perspectives with regard to who the decision maker actually should have been. The majority of the patient participants perceived that the physicians made the decision for them. This belief ran somewhat contrary to many physicians’ statements that it was ultimately the patients who made the treatment decision. This is an interesting finding, and one that requires further investigation. In patients who were in shock at the time of diagnosis, it is conceivable that the physicians presented several options to patients, who were unable to recall the options that were offered due to difficulty processing so much information. It is also possible that when physicians made a strong recommendation for a particular option, the patients may have 88 perceived that they did not have enough knowledge about the different options and therefore they would leave the decision to their physician, who ultimately made the decision for them. In other qualitative studies in men diagnosed with prostate cancer48 and women with ovarian cancer,43 the treatment decision also seemed to be driven mostly by the physicians, with some patients perceiving themselves as passive recipients of care. Future research should seek to uncover whether physicians can present treatment options with more equipoise and whether patients who desire a more shared or active decisional role can be given the opportunity by their physicians to participate more fully in the actual decision making. Limited time has been reported as one of the barriers to effective treatment decisionmaking.49 The study findings described above have confirmed that this negative aspect of treatment decision making was pervasive. Both patient and physician participants acknowledged that the time allotted for treatment decision-making discussions was limited and not sufficient to fostering the kind of discussion of options needed to reach a tailored or “best” treatment decision. The onus is with the physician to provide patients ample time to process the treatment options that they are offering to their patients. Poor quality patientphysician communication has been identified as one of the challenges in treatment decision making50 and is also an area that requires further investigation. Studies that can guide administrative policies on adequate time allocations, in terms of treatment decision encounters, would be very beneficial not only for the patient but also for the physician. Past health experiences and actual experience with myeloma therapy were found to be influential in the treatment considerations by patient participants. These findings have been previously reported in studies conducted in older women (≥70 years of age) with breast cancer. For example, Husain and colleagues42 reported that two women in their study 89 strongly preferred a specific treatment based on their previous health care experience. The researchers found that these women did not consider the treatment information provided by their clinicians; instead they simply requested a specific treatment based on their personal experience with breast cancer therapy. Perceived side effects and actual experience with chemotherapy were also reported as influential factors in the treatment decisions by women with breast cancer. Kreling and colleagues51 have found that women who underwent chemotherapy experienced side effects that were more uncomfortable than what they had expected with one subject who eventually requested a change in her chemotherapy regimen due to the devastating effect of alopecia on her psychological health. In this study, family opinions and opinions of others including a second physician opinion have been considered by older myeloma patients as having an influence in their treatment decisions. Other treatment decision making studies in patients with prostate,13 ovarian,43 breast,42, 51 and colorectal cancer44 have also found that these factors influence treatment decisions. The issues of therapy cost and its eventual impact on personal finances have been described previously by Kutner et al.44 In this study, some patient participants related how they made sure that their insurance covered the treatment they were going to have so as to avoid any negative impact on their personal finances. Some of the participants took into account the actual co-pays and out-of-pocket cost when they made their treatment decisions. The physician’s expertise and practice type have been previously reported as having an influence in treatment selection. For example, one study52 found that Hodgkin disease experts are more likely to individualize patient’s therapy than non-Hodgkin expert physicians and academic physicians are more likely to choose combined modality therapy (CMT) over 90 radiation therapy or chemotherapy alone. Similarly, in patients with LPC, a survey showed that urologists tend to favor surgery while radiation oncologists tend to favor radiation therapy over surgery.53 In an international survey, gastroenterologists tend to favor surgery for the management of gastric lymphoma, while hematologists and oncologists are more inclined to favor conservative therapy.54 In this study, the majority of physician participants (N=8) worked in a myeloma practice with a strong stem cell transplant program, which in effect had a clear influence on treatment decision making as far as screening patients for autologous HSCT eligibility. This study showed that 9 out 10 physician participants screened their patients for HSCT eligibility. There was only one physician participant who did not mention screening for HSCT, a finding that could be explained by the context of the physician interview, which unintentionally drifted towards the discussion of non-transplant treatment options. This small study was exploratory and, therefore, generalizability is inherently limited. The study sample was primarily composed of Caucasian men and women with relatively high incomes and high levels of education, and only the first 6 months after the diagnosis were examined. Thus, the study findings may not be applicable to African American and Hispanic patients or those patients who are beyond 6 months of diagnosis. In addition, the findings may not be applicable to younger patients (<60 years of age) newly diagnosed with myeloma, since they were not included in this study. This younger group may have a different or differently weighted set of TDM influencing factors. In other words, younger patients (≤60 years of age) newly diagnosed with myeloma may have a very different perspective on treatment decision making than their older counterparts. Despite its 91 limitations, this study uncovers new personal and contextual factors influencing treatment decisions. Further studies are recommended to validate these new findings. Conclusion Older myeloma patients (≥60 years of age) consider actual experience with myeloma therapy, physician’s opinion, personal beliefs and values, family opinion, family burden, social support, insurance and convenience of therapy as influential factors in their treatment decisions. Physicians treating older patients with myeloma consider the patient’s comorbidities, performance status, supportive care requirements, their own personal beliefs and values, patient’s medical and clinical factors, patient’s context, family opinion and patient’s treatment preference as having an influence on their treatment decisions. It is critical that patients are given the opportunity to choose the best possible treatment for their myeloma within the limits of their personal, social, and medical contexts. 92 References 1. Renshaw C, Ketley N, Moller H, et al. Trends in the incidence and survival of multiple myeloma in South East England 1985-2004. BMC Cancer. 2010;10:74. 2. Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin. 2010;60(5):277-300. 3. Waxman AJ, Mink PJ, Devesa SS, et al. Racial disparities in incidence and outcome in multiple myeloma: a population-based study. Blood. 2010;116(25):5501-5506. 4. Landgren O, Weiss BM. Patterns of monoclonal gammopathy of undetermined significance and multiple myeloma in various ethnic/racial groups: support for genetic factors in pathogenesis. Leukemia. 2009;23(10):1691-1697. 5. Turesson I, Velez R, Kristinsson SY, et al. Patterns of multiple myeloma during the past 5 decades: stable incidence rates for all age groups in the population but rapidly changing age distribution in the clinic. Mayo Clin Proc. 2010;85(3):225-230. 6. Mehta J, Cavo M, Singhal S. How I treat elderly patients with myeloma. Blood. 2010;116(13):2215-2223. 7. Koreth J, Cutler CS, Djulbegovic B, et al. High-dose therapy with single autologous transplantation versus chemotherapy for newly diagnosed multiple myeloma: a systematic review and meta-analysis of randomized controlled trials. Biol Blood Marrow Transplant. 2007;13(2):183-196. 8. Barlogie B, Kyle RA, Anderson KC, et al. Standard chemotherapy compared with high-dose chemoradiotherapy for multiple myeloma: final results of phase III US Intergroup Trial S9321. J Clin Oncol. 2006;24(6):929-936. 9. Kyle RA, Rajkumar SV. Multiple myeloma. Blood. 2008;111(6):2962-2972. 93 10. Blade J, Rosinol L, Cibeira MT, et al. Hematopoietic stem cell transplantation for multiple myeloma beyond 2010. Blood. 2010;115(18):3655-3663. 11. Ludwig H, Beksac M, Blade J, et al. Current multiple myeloma treatment strategies with novel agents: a European perspective. Oncologist. 2010;15(1):6-25. 12. National Comprehensive Cancer Network. The complete library of NCCN clinical practice guidelines in oncology: multiple myeloma [CD-ROM]. 2011. Fort Washington, PA. 13. Berry DL, Ellis WJ, Woods NF, et al. Treatment decision-making by men with localized prostate cancer: the influence of personal factors. Urol Oncol. 2003;21(2):93-100. 14. Zeliadt SB, Moinpour CM, Blough DK, et al. Preliminary treatment considerations among men with newly diagnosed prostate cancer. Am J Manag Care. 2010;16(5):e121-130. 15. Schroen AT, Brenin DR. Breast cancer treatment beliefs and influences among surgeons in areas of scientific uncertainty. Am J Surg. 2010;199(4):491-499. 16. Rogers SO, Jr., Gray SW, Landrum MB, et al. Variations in surgeon treatment recommendations for lobectomy in early-stage non-small-cell lung cancer by patient age and comorbidity. Ann Surg Oncol. 2010;17(6):1581-1588. 17. Wasif N, Ye X, Giuliano AE. Survey of ASCO members on management of sentinel node micrometastases in breast cancer: variation in treatment recommendations according to specialty. Ann Surg Oncol. 2009;16(9):2442-2449. 94 18. Stewart AK, Bergsagel PL, Greipp PR, et al. A practical guide to defining high-risk myeloma for clinical trials, patient counseling and choice of therapy. Leukemia. 2007;21(3):529-534. 19. Dispenzieri A, Rajkumar SV, Gertz MA, et al. Treatment of newly diagnosed multiple myeloma based on Mayo Stratification of Myeloma and Risk-adapted Therapy (mSMART): consensus statement. Mayo Clin Proc. 2007;82(3):323-341. 20. Palumbo A, Rajkumar SV. Multiple myeloma: chemotherapy or transplantation in the era of new drugs. Eur J Haematol. 2010;84(5):379-390. 21. Dingli D, Rajkumar SV. How best to use new therapies in multiple myeloma. Blood Rev. 2010;24(3):91-100. 22. Reece DE. Recent trends in the management of newly diagnosed multiple myeloma. Curr Opin Hematol. 2009;16(4):306-312. 23. Corso A, Varettoni M. The impact of new emerging drugs in the treatment of multiple myeloma: is there still a role for PBSC transplantation? Curr Stem Cell Res Ther. 2007;2(1):1-11. 24. Berry DL, Ellis WJ, Russell KJ, et al. Factors that predict treatment choice and satisfaction with the decision in men with localized prostate cancer. Clin Genitourin Cancer. 2006;5(3):219-226. 25. Bailey C, Corner J, Addington-Hall J, et al. Treatment decisions in older patients with colorectal cancer: the role of age and multidimensional function. Eur J Cancer Care (Engl). 2003;12(3):257-262. 26. Schrag D, Cramer LD, Bach PB, et al. Age and adjuvant chemotherapy use after surgery for stage III colon cancer. J Natl Cancer Inst. 2001;93(11):850-857. 95 27. Hurria A, Leung D, Trainor K, et al. Factors influencing treatment patterns of breast cancer patients age 75 and older. Crit Rev Oncol Hematol. 2003;46(2):121-126. 28. Taylor WC, Muss HB. Adjuvant chemotherapy of breast cancer in the older patient. Oncology (Williston Park). 2010;24(7):608-613. 29. Moore M, Gibbs P. Adjuvant chemotherapy use among older patients with stage III colon cancer. JAMA. 2010;303(23):2353; author reply 2353-2354. 30. Wan-Chow-Wah D, Monette J, Monette M, et al. Difficulties in decision making regarding chemotherapy for older cancer patients: a census of cancer physicians. Crit Rev Oncol Hematol. 2010. Epub ahead of print. 31. Repetto L. Greater risks of chemotherapy toxicity in elderly patients with cancer. J Support Oncol. 2003;1(4 Suppl 2):18-24. 32. Repetto L, Comandini D, Mammoliti S. Life expectancy, comorbidity and quality of life: the treatment equation in the older cancer patients. Crit Rev Oncol Hematol. 2001;37(2):147-152. 33. Balducci L. ESH-SIOG International Conference on Haematological Malignancies in the Elderly. Expert Rev Hematol. 2010;3(6):675-677. 34. Balducci L. Management of cancer in the elderly. Oncology (Williston Park). 2006;20(2):135-143; discussion 144, 146, 151-132. 35. Martin VC, Roberto KA. Assessing the stability of values and health care preferences of older adults: A long-term comparison. J Gerontol Nurs. 2006;32(11):23-33. 36. Sekeres MA, Stone RM, Zahrieh D, et al. Decision-making and quality of life in older adults with acute myeloid leukemia or advanced myelodysplastic syndrome. Leukemia. 2004;18(4):809-816. 96 37. Hsieh HF, Shannon SE. Three approaches to qualitative content analysis. Qual Health Res. 2005;15(9):1277-1288. 38. QSR International Ltd. NVivo 8 Software. 2008. Available at http://www.qsrinternational.com/FileResourceHandler.ashx/RelatedDocuments/Docu mentFile/289/NVivo8-Getting-Started-Guide.pdf. Accessed on February 12, 2011. 39. Hill CE. A guide to conducting consensual qualitative research. The Counseling Psychologist. 1997;25(4):517-572. 40. Krippendorff K. Reliability. Content analysis: an introduction to its methodology. Beverly Hills, CA: Sage Publications; 1980:129-154. 41. Berry DL, Maliski SL, Ellis WJ. Qualitative research techniques. In: Penson DF, Wei JT, eds. Clinical Research Methods for Surgeons. Totowa, NJ: Human Press, Inc; 2006:297-310. 42. Husain LS, Collins K, Reed M, et al. Choices in cancer treatment: a qualitative study of the older women's (>70 years) perspective. Psychooncology. 2008;17(4):410-416. 43. Elit L, Charles C, Gold I, et al. Women's perceptions about treatment decision making for ovarian cancer. Gynecol Oncol. 2003;88(2):89-95. 44. Kutner JS, Vu KO, Prindiville SA, et al. Patient age and cancer treatment decisions. Patient and physician views. Cancer Pract. 2000;8(3):114-119. 45. Attal M, Harousseau JL. Role of autologous stem-cell transplantation in multiple myeloma. Best Pract Res Clin Haematol. 2007;20(4):747-759. 46. Child JA, Morgan GJ, Davies FE, et al. High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. New Engl J Med. 2003;348(19):1875-1883. 97 47. Klepin HD, Hurd DD. Autologous transplantation in elderly patients with multiple myeloma: are we asking the right questions? Bone Marrow Transplant. 2006;38(9):585-592. 48. Cohen H, Britten N. Who decides about prostate cancer treatment? A qualitative study. Fam Pract. 2003;20(6):724-729. 49. Shepherd HL, Tattersall MH, Butow PN. Physician-identified factors affecting patient participation in reaching treatment decisions. J Clin Oncol. 2008;26(10):1724-1731. 50. Blank T, Graves K, Sepucha K, et al. Understanding treatment decision making: contexts, commonalities, complexities, and challenges. Ann Behav Med. 2006;32(3):211-217. 51. Kreling B, Figueiredo MI, Sheppard VL, et al. A qualitative study of factors affecting chemotherapy use in older women with breast cancer: barriers, promoters, and implications for intervention. Psychooncology. 2006;15(12):1065-1076. 52. Ng AK, Li S, Neuberg D, et al. Factors influencing treatment recommendations in early-stage Hodgkin's disease: a survey of physicians. Ann Oncol. 2004;15(2):261269. 53. Fowler FJ, Jr., McNaughton Collins M, Albertsen PC, et al. Comparison of recommendations by urologists and radiation oncologists for treatment of clinically localized prostate cancer. JAMA. 2000;283(24):3217-3222. 54. de Jong D, Aleman BM, Taal BG, et al. Controversies and consensus in the diagnosis, work-up and treatment of gastric lymphoma: an international survey. Ann Oncol. 1999;10(3):275-280. 98 CHAPTER IV Decisional Role Preferences in Older Adults Newly Diagnosed with Myeloma Introduction In the past three decades, treatment decision making (TDM) studies have focused on decisional control preferences, with most studies conducted in breast and prostate cancer samples. As reviewed by Tariman et al., these studies have shown an increasing ratio of patients being interested in more active TDM participation.1 In the 1980s, decision-making researchers examined decisional control preferences as a dichotomous variable rather a continuous or ordinal variable.2-4 Through an extensive field study of how treatment decisions are made for patients with life-threatening illnesses such as cancer, Degner and Beaton5 uncovered 4 patterns of control over decision making.6 These observations led to the formulation of the control preferences construct and the validation of the hypothesized psychological dimension that patients have differing preferences about keeping control over TDM, sharing control with their physician and relinquishing control to their physician.7 Early decision making studies from the late 1990s and early 2000s in older adults with cancer showed that most preferred a specific option when presented with a variety of treatment options for cancer8 and end-of-life preferences.9 Moreover, two systematic reviews examining decisional control preferences have shown that patient preferences for involvement in TDM are highly variable and that these decisional preferences change as the disease progresses.1, 4 In order to meet and facilitate the patient’s preferred level of participation during TDM, interventional studies geared toward increasing a patient’s decisional satisfaction, reducing decisional conflict, and preventing anxiety and depression 99 related to TDM have been steadily increasing in numbers,10-26 with some studies targeting the elderly cancer patient population.27-30 The growing interest in direct assessment of patient preferences for all components of decision making,31-41 coupled with a predicted shift away from paternalistic decisions as baby boomers age,41 present substantial opportunities for improving patient care and clinical outcomes in the area of decision making. These opportunities are particularly true in the older adult cancer patient population, which is often understudied or underrepresented in clinical trials. Increased patient participation in TDM has been associated with positive clinical outcomes such as a greater level of patient satisfaction with decisions and better psychological adjustment (e.g., less post-decision anxiety and depression) in patients.4, 42 However, the preferred level of participation in treatment decision making for patients diagnosed with myeloma has not been previously studied. Moreover, the impact of older adult patients’ participation in decision making processes is unknown, and the patient and physician factors associated with participation in decision making specific to this population have not been previously explored. Theoretical Framework Degner and Beaton’s Patterns of Decision Making5 provides the theoretical framework for this study. These patterns encompass the various levels of patient participation in decision making and are patient-centered, directly eliciting decisional role preferences from the patient’s perspective. According to the model, four decision-making patterns may occur. A provider-controlled decision-making pattern emerges when patients decline to become involved in selecting their own treatment, even when urged to do so by 100 the physician. In this case, the patient is essentially saying: It’s up to you, doctor; you’re the expert. On the other hand, a patient-controlled decision-making pattern occurs when patients make it clear that they are the ones who will make the decisions.5 When patients want a discussion of options with their physician and to think about the options prior to making the final decision with their physician, a jointly-controlled decision-making pattern occurs. Finally, when patients are incapable of making treatment decisions and the family makes decisions for them, a family-controlled decision-making pattern emerges.5 Table 1 illustrates the various roles patients can play during treatment decision making. This framework of decisional role patterns was developed based on a 4-year qualitative study into decision-making roles in life-threatening situations such as cancer.5 The control preference scale (CPS) is a measure of decision-making preferences7 and it identifies these preferences on a series of cards. Table 1. Degner and Beaton’s Patterns of Decision Making7 (The Control Preferences Scale) _______________________________________________________________________ Active – Patient-Controlled o I prefer to make the final treatment decision (Card A). o I prefer to make the final treatment decision after seriously considering my doctor’s opinion (Card B). Collaborative – Jointly controlled o I prefer that my doctor and I share responsibility for deciding which treatment is best (Card C). Passive – Provider-controlled o I prefer my doctor to make the final treatment decision, but only after my doctor have seriously considered my opinion (Card D). o I prefer to leave all treatment decisions to my doctor (Card E). Family-Controlled o The family makes final decisions for the patient, who is incapacitated. _______________________________________________________________________ 101 Purpose The purpose of the study was to describe the preferences for participation in decision making of patients newly diagnosed with multiple myeloma and to explore the association between sociodemographic variables and decisional role preferences. Methods Design and Sample A cross-sectional survey approach was employed involving administration of the Control Preferences Scale during a one-time semi-structured interview. The convenience sample consisted of 20 older adults (60 years of age and above) referred to the Seattle Cancer Care Alliance (SCCA) or the Northwestern University Myeloma Program (NUMP) by hematologists/oncologists in the greater Seattle or Chicago areas, respectively. Eligibility criteria included older adults who were (a) 60 years of age and above, (b) newly diagnosed with multiple myeloma, (c) able to read and write English, and (d) able to give informed consent. Instrument The CPS is a measure of decisional role preferences using a card-sort technique that has two sets of five cards each. Each card describes a different role in decision making and is illustrated with a sketch of characters (doctor/patient) representing their different roles in decision making. The first set of five cards illustrates possible roles that the patient could assume, ranging from the patient selecting his or her own treatment (cards A and B) through a collaborative role model (card C) to a scenario where the physician makes the decision (cards D and E) as shown in Appendix A. There were no cases of family controlled decisionmaking in the current study since the eligibility criteria excluded anyone in this category. 102 Patients were asked to complete the card sorts one at a time, comparing each card with every other card in subsets of two, until their entire preference order across the set of five cards was unfolded. The first two cards (B and D) were placed in front of the subject, who was asked to select a preferred card based on a fixed-order presentation (B-D-C-E-A). The preferred card was then placed on top of the non-preferred card. Then the next card (C) was removed from the deck and placed beside the new stack of two cards. The participant was asked to compare the card (C) to the most preferred card. If the patient still preferred the previous card over the new one, the previous card was flipped over and the new card was compared to the next one on a fixed-order basis as described above. Once the preferred card was selected, the next card (E) was compared to the preferred card, and so on until the last card (A) was compared to the previously preferred card. According to Degner et al., this process unfolds the patient’s decisional role preference.7 The CPS offers an easy and quick way to elicit patient preferences about participating in health care decision making.7 This scale has been found to be valid in the measurement of decisional role preferences in patients newly diagnosed with various types of cancer,43 including men diagnosed with localized prostate cancer44, 45 and their partners,45 patients diagnosed with colorectal cancer,46 breast cancer patients at any point of the disease trajectory47 and older adults with breast cancer diagnosed in the past 18 months.48 Permission to use the CPS scale for this study was obtained prior to study design. Recruitment Procedures After the researcher obtained approval from the University of Washington (UW) and Northwestern University (NU) Human Subjects Divisions, older adults newly diagnosed with myeloma were recruited to participate in the study. The researcher made every effort to 103 recruit from both university- and community-based practices in order to include a diversity of study participants. Adults ages 60 and older who had an appointment at the hematology or transplant services of SCCA and were found to be eligible for the study were recruited by mail using a recruitment flyer. At NUMP, the researcher personally approached potential participants (direct approach) and introduced the study to gauge their interest in participation. A review of clinic schedules at SCCA and NUMP was conducted weekly to identify potential study participants. One other UW-affiliated clinic (where myeloma patients were seen) was checked weekly for potential study participants. To establish the decisional role preferences, a card-sort technique was employed, using the fixed-order presentation described by Degner and colleagues.7 In this study, the CPS was administered by the researcher during a semi-structured interview conducted in designated research-related conference rooms at SCCA and NUMP. These rooms were strictly assigned for research use only and met the human subject division’s standard for patient privacy. If a patient wanted the interview to be conducted at a later time, a one-week period following the appointment was allowed for re-scheduling. Moreover, if a patient wanted the interview to be conducted at his/her home, the researcher conducted the interview at the subject’s home, as requested. Analysis Coombs’ Unfolding Analysis Data from the CPS scale were analyzed using the Statistical Analysis System® (Cary, NC) computer program syntax developed by Sloan and Yeung.49, 50 This analysis was based on a scaling model developed by Coombs, termed the unfolding theory.51 According to 104 Degner and colleagues7 this psychological scaling model is based on the assumption that “an individual’s preference corresponds to an ideal point on a continuum, and that this ideal point can be derived by presenting successive paired comparisons of stimuli that fall along the continuum (p.25).” The unfolding theory holds that for any given hypothesized scale or metric, only 11 subsets of the 120 possible permutations of the five decisional role cards will be transitive. These 11 transitive responses range from ABCDE to EDCBA and they include ABCDE, BACDE, BCADE, BCDAE, CBDAE, CDBAE, CDBEA, CDEBA, DCEBA, DECBA and EDCBA. “Transitivity” means that an individual’s response falls within the 11 possible valid permutations and the individual’s preference for each of the paired comparisons of stimuli is consistent with the hypothesized A to E psychological continuum.7 The reliability of the scale is established when 50% plus one of the experimental participants’ preferences were transitive, falling along the hypothesized scale (i.e., the ABCDE psychological continuum). Triangulation of Qualitative and Quantitative Data Across-method triangulation,52 a form of methods triangulation was employed for cross validation of the data pertaining to patients’ decisional role preferences. Patients’ verbal descriptions of their desired level of participation (as elicited from the interview) and the patients’ preferences for participation (as measured by the CPS card sort) were compared. This approach collected rich, detailed information from the participants regarding their perspectives on decisional role preferences, which were then compared to the description of the CPS cards. Association Analyses 105 The differences in decisional role preferences were examined using dichotomous categories of gender (male versus female), age (<70 years versus ≥70 years), education (less than 4 years of college versus ≥4 years of college), relationship status (single versus partnered), income (≤$55,000 versus >$55,000), and employment status (retired versus nonretired) were compared using simple analysis of variance (ANOVA). Spearman rank-order correlations were used to determine the relationship between the ordinal CPS score and the respondent’s age, education, and income variables. Given the results of the bivariate analysis and the small sample size, multivariate analyses were not carried out. Results A total of seventy-nine potential participants was recruited by mail at SCCA from October 2009 through July 2010. Of these, 14 (17.7 %) participants responded and all participated in the study. At NUMP, the researcher identified six potential participants and approached them about participation. All six potential participants agreed to participate. Informed consent was obtained from all study participants before collecting any data. Table 2 presents sociodemographic characteristics of the study participants. The participants’ mean age was 67.5 years (age range: 60-82 years). The majority of participants in this sample were Caucasian (90%); female (60%), married or partnered (60%), retired (65%), had at least a 2-year college education (75%) and had an income >$35,000 (75%). 106 Table 2. Sociodemographic Characteristics of the Sample Variable Age (mean, 67.5 years) 60-70 70-82 Gender Male Female Race White Asian American Indian/Native Alaskan Native Employment Status Full time Working on medical leave Not working Retired Student Personal Relationship Status Single Married or partnered Divorced Widowed Highest Level of Education 9th – 12th grade 2 years of college 4 years of college Graduate degree Annual Household Income $18,000 or less $18,000 to $35,000 $35,001 to $55,000 $55,001 to $85,000 $85,001 and above N % 14 6 70 30 8 12 40 60 18 1 1 90 5 5 2 2 2 13 1 10 10 10 65 5 2 12 5 1 10 60 25 5 5 2 10 3 25 10 50 15 3 2 5 5 5 15 10 25 25 25 Unfolding Analysis As illustrated in Table 3, the hypothesized ABCDE metric has more than 50% of the respondents’ answers corresponding to one of its 11 transitive preference orders. This means that the data show support for an underlying dominant dimension of control, ranging from 107 keeping control (active - cards A and B) through collaboration (sharing- card C) to giving away control (passive- cards D and E). The DCBAE metric also has more than 50% of the respondents’ answers corresponding to one of its 11 transitive preference orders. This means that a second, competing model of dichotomous preference (shared, card C, and active, cards B and A) is seen in this group of older adults newly diagnosed with myeloma. The table also shows the other competing scales under the column NAME (CBADE, ACBDE, etc.), which did not meet the reliability criterion of 50% plus 1 of the experimental participants’ preferences corresponding to one of the 11 transitive preference orders. Table 3 summarizes the participants’ valid responses (valid) and invalid response (invalid) and their corresponding percentages (valper and invalper, respectively). There was no reversal found since no participant selected an extreme role represented by card A or card E. Figure 1 shows the distribution of respondents’ preferences falling on and off the transitive response of the ABCDE metric. Table 3. Rank Ordering of Competing Scale Models SCALE 1 60 47 7 58 15 31 33 55 56 CONTROL PREFERENCES SCALING SUMMARY FOR SCORING NAME VALID VALPER INVALID INVALPER ABCDE DCBAE CBADE ACBDE DBCAE ADCBE BCADE BCDAE DABCE DACBE 14 14 9 7 7 6 6 6 5 4 70 70 45 35 35 30 30 30 25 20 6 6 11 13 13 14 14 14 15 16 30 30 55 65 65 70 70 70 75 80 EMCELL REVERSAL 3 6 8 8 8 8 9 8 9 10 N N N N N N N N N N 108 Figure 1. Distribution of Respondents On and Off ABCDE Metric Decisional Role Preferences An examination of the distribution of preferences based on the first card in the preference order indicated that 55% (N=11) of the participants preferred a shared role with the physician and that 40% (N=8) preferred to make the decisions after seriously considering the opinion of their physician (see Table 4). Only one participant preferred to relinquish the decision to the doctor as long as the doctor considered the patient’s preferences. No individual chose card A or E, which are the extremes of all the preference choices. Overall, the percentage of the patients wanting to have some kind of control over the treatment decision is very high at 95% (N=19). 109 Table 4. Distribution of Decisional Role Preferences Preferred Valid Cumulative Frequency Percent Role Percent Percent B (Active) 8 40.0 40.0 40.0 C (Shared) 11 55.0 55.0 95.0 D (Passive) 1 5.0 5.0 100.0 20 100.0 100.0 Total Sociodemographic Variables and Decisional Role Preferences There were no statistically significant differences in decisional role preferences across dichotomous categories of gender (male versus female), age (<70 years versus ≥70 years), education (less than 4 years of college versus greater than 4 years of college), partner status (single versus partnered), income (≤$55,000 versus >$55,000), and employment status (retired versus non-retired) using ANOVA. Given the small sample size, we explored only simple bivariate differences between decisional role preference and these other factors. The results of the bivariate analysis and the small sample size did not warrant further multivariate analyses. There are also no statistically significant correlations between decisional role preference and age, education, or income, respectively. The distribution of decisional role preferences by age group, education, and income are shown in Table 5, 6, and 7, respectively. 110 Table 5. Decisional Role Preferences by Age Preferred Role 60-65 B (Active) C (Shared) D (Passive) 66-70 4 6 1 11 2 1 0 3 Age Group 71-75 76-80 81 and above 1 1 0 1 1 2 0 0 0 2 2 2 Total 8 11 1 20 Total Table 6. Decisional Role Preferences by Education Highest Level of Education Preferred Role 9th to 12th grade B (Active) C (Shared) D (Passive) Total 2-year college 2 3 0 5 4-year college 0 2 0 2 Graduate degree 5 5 0 10 Total 1 1 1 3 8 11 1 20 Table 7. Decisional Role Preferences by Income Annual Household Income Preferred Role $18,000 $18,000 to or less $35,000 $35,001 $55,001 to $85,001 to $85,000 and above $55,000 Total B (Active) 1 0 1 4 2 8 C (Shared) 2 2 4 1 2 11 D (Passive) 0 0 0 0 1 1 Total 3 2 5 5 5 20 111 Quantitative and Qualitative Decisional Role Preferences Aside from using the CPS card-sort to measure the patients’ preferences for decisional participation, patients were also asked to describe the decisional role that they prefer from the CPS card. Table 8 illustrates the patient decisional role preferences using the CPS card, the decisional category, and the patient’s own description of participation. Table 8. Patient’s Decisional Role Preference, Category, and Description Subject ID First Card in CPS Decisional Category A B Active “Well, we knew that it was incurable, and the medical community nationwide seemed to be taking the transplant approach to improve the possibilities of longevity. So we were impressed with the facility and all of the staff and have decided to go ahead in that direction.” B C Shared “I’d like to be well informed of my choices, on both the pros and cons of those choices, in language I can understand, so that I can help participate in making the choice.” Patient’s Description of Preferred Decisional Role Description Matches with Decisional Category Yes Yes 112 Table 8 continued Subject ID First Card in CPS Decisional Category Patient’s Description of Preferred Decisional Role C C Shared “Well, I like to have the best possible treatment plans to cure my disease, and knowing my doctor, I was confident at that time to listen to her opinion, and we made a decision collectively to further my treatment.” D C Shared “I would like to go over the pros and cons of therapy. I see them in my life for me and then make that decision with the doctor as far as what the doctor feels after the doctor heard where I was coming from. The doctor knows what would be the best for me in this situation because the doctor has the overall picture and I only have pieces.” Description Matches with Decisional Category Yes Yes 113 Table 8 continued Description Matches with Decisional Category No Subject ID First Card in CPS Decisional Category E B Active “I want to know, I like to understand, really, why something is being done. Not that I truly would understand it as a lay person, but I want to understand the logic for doing something and I do want to understand the potential for being successful and risk factors, you know, because I want to use that to gauge how fast I want to live my life.” G B Active “So it was neutral at first, and then as some of the shock wore off and some of the reality came in, I started participating more in my treatment.” Yes H C Shared “The fact that before treatments are started, I know what it is going to be, and if there is for some reason a drug that I don’t feel I could take, then I still have a right to say no to that.” Yes Patient’s Description of Preferred Decisional Role 114 Table 8 continued Description Matches with Decisional Category Yes Subject ID First Card in CPS Decisional Category Patient’s Description of Preferred Decisional Role I D Passive “I definitely want to be involved in the decision, but knowing that the doctor knows more than I do about the treatments that are available and which one I’m best suited for, I would go with the doctor’s opinion after I’ve heard what the options are and discussed them.” J B Active “I would like to have full involvement. I will listen to what the doctor says or what he feels, because I feel he has that knowledge. And I probably would take his recommendation, but I would make the decision myself.” Yes K C Shared “My oncologist seems to think that acupuncture and massage are all fine, but those are things that I’ve explored myself. So I take his advice, and then I do my other kinds of things that are alternative sorts of things, too.” Yes 115 Table 8 continued Description Matches with Decisional Category Yes Subject ID First Card in CPS Decisional Category L B Active “I write things down and I try to get as many opinions as possible. And of course I think the doctor’s opinion about what to do is 90% of what it is. But I want to understand, if decisions are being made on my behalf, why they're being made.” M C Shared “Well, my preference is and it has been so far is shared involvement with the doctor and also a key to it has been the ability to be able to get a second opinion, so I can have two experts look at the situation.” Yes N B Active “My wife was heavily involved in making the decision, too. Since she was very much affected by it, so together we made the decision. I probably had a little more influence than her, but her opinion was considered as well.” Yes Patient’s Description of Preferred Decisional Role 116 Table 8 continued Description Matches with Decisional Category No Subject ID First Card in CPS Decisional Category O C Shared “I want to know. I am very, very nosey that way. I want to know.” P C Shared “I want her [my doctor] to see how I’m doing and see what condition I am and how I’m progressing, you know; worse or better, then, make her decision based on that.” No Q C Shared “I take the input from what I've gotten back from the tests that my doctor sends me. But that wasn't good enough for me because I wanted a second opinion.” Yes R C Shared “Now that I’ve kind of had a chance to step back and have a more sober, view of it and more objective view, I feel that I’m in a better frame of mind, if you would, to maybe look at the options and discuss this in a more objective manner.” Yes Patient’s Description of Preferred Decisional Role 117 Table 8 continued Description Matches with Decisional Category Yes Subject ID First Card in CPS Decisional Category S C Shared “I want to know about things. I'm curious. I want to know as much as I can. And then with the help of my husband and my kids, make a decision.” T B Active “Well, I will make my decision along with my husband at that point on which is best for us as a family, and we rely upon our doctor's medical advice to lead us to a conclusion.” Yes U B Active “I ask her [my doctor] everything I can think of when we meet. I listen to what she has to tell me. If the decision is something clear enough that I can make it immediately or if I need to make it immediately, I do.” Yes Patient’s Description of Preferred Decisional Role Note: Study ID numbers have been changed to ensure full protection of participants confidentiality. Discussion In this study of decisional role preferences in older adults newly diagnosed with myeloma, a high percentage (95%) of participants wanted to have some control (shared and active roles) of the treatment decisions with only 1 subject (5%) expressing preference for a passive role. This finding is contrary to previous reports that older adults are passive recipients of medical care.43, 53-57 Perhaps the impact of advanced age on relinquishing 118 decisional control to physicians is moderated by the higher education and higher income profile of the study participants; these are variables previously reported as having strong correlations with more decisional control.43, 48, 58, 59 Alternatively, myeloma patients may have a different profile of decisional role preferences from patients with other cancers. The findings of the study are consistent with the most recent findings reported in studies from the United Kingdom60 and Canada,25, 44 where research showed increasing numbers of patients wanting to have some control of treatment decisions—as high as 92-93% in some studies. It would be interesting to see a consistent trend of patient’s decisional role preferences moving towards more participation in the near future. Based on interview transcripts from the current study, it appears that patients’ preferred decisional roles elicited from the use of the CPS scale generally matched with their individual descriptions of decisional role preference. This finding supports the hypothesized construct that patients have various decisional preferences about keeping, sharing, or relinquishing control over treatment decisions. It should be noted, however, that we did not measure the degree of congruence between patients’ desired role and actual role during the TDM process. Participants in this study demonstrated a strong desire to participate in the decisionmaking process, though they may not have had a full understanding of myeloma and its treatment options. It is possible that the participants have unmet information needs leading to more active participation. In their descriptions of role preferences, participants in this study reported seeking information from various sources and some participants identified their doctors as the primary source to explain the different treatment options available to them. 119 This finding has strong implications for physicians regarding how they provide information to patients. The emergence of a second valid metric in Coombs’ unfolding analysis warrants further exploration. The participants had a nearly 50/50 distribution between shared and active decisional role preferences. Only 1 subject chose to be a passive recipient of care. This is clearly a trend seen in the Western societies, where health care consumerism is on the rise.61-63 One could theorize that the former paternalistic model of physician-patient relationship is losing ground, as suggested by Rosenstein in the mid-1980s.64 Patient preferences have always tended to fall into a dichotomy of preferences. The shift in decisional preference toward either a shared or active role for patients has not been reported before. In the past, patient preferences tended to fall into either the “active” or “passive” category.2, 3 It is also possible that the majority of the participants in this study included patients who are not too sick from the myeloma or from their chemotherapy, and therefore allows them to be more active with their treatment decisions. There are limitations of this study primarily related to sample size and demographics. The small sample consisted of mostly Caucasians who were relatively highly educated and high-income individuals. In addition, the majority of participants (85%) were receiving care at a university-based comprehensive cancer center. The small sample size and lack of a diverse sample limit the generalizability of study findings. We were unable to make more meaningful comparisons of differences in decisional role preferences using dichotomous sociodemographic variables and were also unable to examine associations of decisional role preferences based on specific combinations of sociodemographic variables using multivariate analysis. Moreover, since this is a cross-sectional study, the findings may not be applicable to 120 myeloma patients who are beyond 6 months of diagnosis. These limitations should be addressed in future research. Additionally, further study using a longitudinal approach is needed to better describe the stability or change in patients’ decisional role preference over time. Implications for Practice The study findings suggest that patients diagnosed with myeloma do want to participate in the treatment decision-making process. Although myeloma is complex and not easy to understand for laypersons, these findings indicate that patients still want to learn as much as they reasonably can about the disease and treatment so as to understand the reason why certain treatment options might be better for them than others. The majority of participants also wanted to share the treatment decision with their physicians and/or want to make the decision themselves. Therefore, it is critical for physicians to practice full disclosure of treatment options to their patients so patients can make a truly informed decision. Since a patient’s level of preference for participation is highly variable and is unique in terms of personal meaning, physicians need to elicit the patient’s preference, explore what participation truly means for him/her and facilitate the patient’s decision process. Because more patients now want to participate in TDM, physicians, nurses, and policy makers should support more research that can enhance patient involvement in treatment decision making. Conclusion Older adults newly diagnosed with myeloma are likely to want a role in the treatment decision-making process. Nurses can help patients achieve the level of participation they desire by making sure patients receive disease and treatment-related information and 121 encouraging patients to express their decisional role preference to the physician. Nurses can also convey the patient’s decisional role preferences to the physician. More studies that focus on supporting and involving patients in the decision-making process are needed in order to influence clinical practice and policy in this direction. 122 References 1. Tariman JD, Berry DL, Cochrane B, et al. Preferred and actual participation roles during health care decision making in persons with cancer: a systematic review. Ann Oncol. 2010;21(6):1145-1151. 2. Blanchard CG, Labrecque MS, Ruckdeschel JC, et al. Information and decisionmaking preferences of hospitalized adult cancer patients. Soc Sci Med. 1988;27(11):1139-1145. 3. Cassileth BR, Zupkis RV, Sutton-Smith K, et al. Information and participation preferences among cancer patients. Ann Intern Med. 1980;92(6):832-836. 4. Gaston CM, Mitchell G. Information giving and decision-making in patients with advanced cancer: a systematic review. Soc Sci Med. 2005;61(10):2252-2264. 5. Degner LF, Beaton JI. Life death decisions in health care. New York: Hemisphere Publishing; 1987. 6. Degner LF, Russell CA. Preferences for treatment control among adults with cancer. Res Nurs Health. 1988;11(6):367-374. 7. Degner LF, Sloan JA, Venkatesh P. The Control Preferences Scale. Can J Nurs Res. 1997;29(3):21-43. 8. Mazur DJ, Merz JF. How older patients' treatment preferences are influenced by disclosures about therapeutic uncertainty: surgery versus expectant management for localized prostate cancer. J Am Geriatr Soc. 1996;44(8):934-937. 9. Vig EK, Davenport NA, Pearlman RA. Good deaths, bad deaths, and preferences for the end of life: a qualitative study of geriatric outpatients. J Am Geriatr Soc. 2002;50(9):1541-1548. 123 10. Smith SK, Trevena L, Simpson JM, et al. A decision aid to support informed choices about bowel cancer screening among adults with low education: randomised controlled trial. BMJ. 2010;341:c5370. 11. Evans R, Joseph-Williams N, Edwards A, et al. Supporting informed decision making for prostate specific antigen (PSA) testing on the web: an online randomized controlled trial. J Med Internet Res. 2010;12(3):e27. 12. Caldon LJ, Collins KA, Reed MW, et al. Clinicians' concerns about decision support interventions for patients facing breast cancer surgery options: understanding the challenge of implementing shared decision-making. Health Expect. 2010. Epub ahead of print. 13. Steginga SK, Turner E, Donovan J. The decision-related psychosocial concerns of men with localised prostate cancer: targets for intervention and research. World J Urol. 2008;26(5):469-474. 14. O'Connor AM, Tugwell P, Wells GA, et al. A decision aid for women considering hormone therapy after menopause: decision support framework and evaluation. Patient Educ Couns. 1998;33(3):267-279. 15. Sawka AM, Straus S, Brierley JD, et al. Decision aid on radioactive iodine treatment for early stage papillary thyroid cancer--a randomized controlled trial. Trials. 2010;11:81. 16. O'Connor AM, Stacey D, Rovner D, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2001(3):CD001431. 17. Stacey D, Samant R, Bennett C. Decision making in oncology: a review of patient decision aids to support patient participation. CA Cancer J Clin. 2008;58(5):293-304. 124 18. Molenaar S, Sprangers MA, Rutgers EJ, et al. Decision support for patients with early-stage breast cancer: effects of an interactive breast cancer CDROM on treatment decision, satisfaction, and quality of life. J Clin Oncol. 2001;19(6):16761687. 19. Chiew KS, Shepherd H, Vardy J, et al. Development and evaluation of a decision aid for patients considering first-line chemotherapy for metastatic breast cancer. Health Expect. 2008;11(1):35-45. 20. Wakefield CE, Watts KJ, Meiser B, et al. Development and pilot testing of an online screening decision aid for men with a family history of prostate cancer. Patient Educ Couns. 2011;83(1):64-72. 21. Schroy PC, 3rd, Emmons K, Peters E, et al. The impact of a novel computer-based decision aid on shared decision making for colorectal cancer screening: a randomized trial. Med Decis Making. 2011;31(1):93-107. 22. Murray MA, Stacey D, Wilson KG, et al. Skills training to support patients considering place of end-of-life care: a randomized control trial. J Palliat Care. 2010;26(2):112-121. 23. Jibaja-Weiss ML, Volk RJ, Granchi TS, et al. Entertainment education for breast cancer surgery decisions: A randomized trial among patients with low health literacy. Patient Educ Couns. 2010. Epub ahead of print. 24. Allen JD, Othus MK, Hart A, Jr., et al. A randomized trial of a computer-tailored decision aid to improve prostate cancer screening decisions: results from the take the wheel trial. Cancer Epidemiol Biomarkers Prev. 2010;19(9):2172-2186. 125 25. Davison BJ, Goldenberg SL, Gleave ME, et al. Provision of individualized information to men and their partners to facilitate treatment decision making in prostate cancer. Oncol Nurs Forum. 2003;30(1):107-114. 26. Hack TF, Pickles T, Bultz BD, et al. Impact of providing audiotapes of primary treatment consultations to men with prostate cancer: a multi-site, randomized, controlled trial. Psychooncology. 2007;16(6):543-552. 27. Lewis CL, Golin CE, DeLeon C, et al. A targeted decision aid for the elderly to decide whether to undergo colorectal cancer screening: development and results of an uncontrolled trial. BMC Med Inform Decis Mak. 2010;10:54. 28. van Tol-Geerdink JJ, Leer JW, van Lin EN, et al. Offering a treatment choice in the irradiation of prostate cancer leads to better informed and more active patients, without harm to well-being. Int J Radiat Oncol Biol Phys. 2008;70(2):442-448. 29. Beeker C, Kraft JM, Southwell BG, et al. Colorectal cancer screening in older men and women: qualitative research findings and implications for intervention. J Community Health. 2000;25(3):263-278. 30. Partin MR, Nelson D, Flood AB, et al. Who uses decision aids? Subgroup analyses from a randomized controlled effectiveness trial of two prostate cancer screening decision support interventions. Health Expect. 2006;9(3):285-295. 31. Flynn KE, Smith MA, Vanness D. A typology of preferences for participation in healthcare decision making. Soc Sci Med. 2006;63(5):1158-1169. 32. Beaver K, Booth K. Information needs and decision-making preferences: comparing findings for gynaecological, breast and colorectal cancer. Eur J Oncol Nurs. 2007;11(5):409-416. 126 33. Mancini J, Geneve J, Dalenc F, et al. Decision-making and breast cancer clinical trials: how experience challenges attitudes. Contemp Clin Trials. 2007;28(6):684-694. 34. Nekhlyudov L, Li R, Fletcher SW. Information and involvement preferences of women in their 40s before their first screening mammogram. Arch Intern Med. 2005;165(12):1370-1374. 35. Sabo B, St-Jacques N, Rayson D. The decision-making experience among women diagnosed with stage I and II breast cancer. Breast Cancer Res Treat. 2007;102(1):51-59. 36. Berry DL, Ellis WJ, Woods NF, et al. Treatment decision-making by men with localized prostate cancer: the influence of personal factors. Urol Oncol. 2003;21(2):93-100. 37. Denberg TD, Melhado TV, Steiner JF. Patient treatment preferences in localized prostate carcinoma: The influence of emotion, misconception, and anecdote. Cancer. 2006;107(3):620-630. 38. Kramer KM, Bennett CL, Pickard AS, et al. Patient preferences in prostate cancer: a clinician's guide to understanding health utilities. Clin Prostate Cancer. 2005;4(1):1523. 39. Volk RJ, Cantor SB, Cass AR, et al. Preferences of husbands and wives for outcomes of prostate cancer screening and treatment. J Gen Intern Med. 2004;19(4):339-348. 40. Zeliadt SB, Ramsey SD, Penson DF, et al. Why do men choose one treatment over another?: a review of patient decision making for localized prostate cancer. Cancer. 2006;106(9):1865-1874. 127 41. Pipe TB, Conner K, Dansky K, et al. Perceived treatment involvement in decisionmaking as predictor of decision satisfaction in older adults. SOJNR. 2005;6(4):1-13. 42. Gattellari M, Butow PN, Tattersall MH. Sharing decisions in cancer care. Soc Sci Med. 2001;52(12):1865-1878. 43. Degner LF, Sloan JA. Decision making during serious illness: what role do patients really want to play? J Clin Epidemiol. 1992;45(9):941-950. 44. Davison BJ, Goldenberg SL, Wiens KP, et al. Comparing a generic and individualized information decision support intervention for men newly diagnosed with localized prostate cancer. Cancer Nurs. 2007;30(5):E7-E15. 45. Davison BJ, Gleave ME, Goldenberg SL, et al. Assessing information and decision preferences of men with prostate cancer and their partners. Cancer Nurs. 2002;25(1):42-49. 46. Beaver K, Bogg J, Luker KA. Decision-making role preferences and information needs: a comparison of colorectal and breast cancer. Health Expect. 1999;2(4):266276. 47. Degner LF, Kristjanson LJ, Bowman D, et al. Information needs and decisional preferences in women with breast cancer. JAMA. 1997;277(18):1485-1492. 48. Wallberg B, Michelson H, Nystedt M, et al. Information needs and preferences for participation in treatment decisions among Swedish breast cancer patients. Acta Oncol. 2000;39(4):467-476. 49. Sloan JA, Yeung A. A Manual for Implementing Coombs' Unidimensional Unfolding Model for Paired Comparisons Data. Manitoba Nursing Research Institute Technical Report #12. University of Manitoba. 1994. 128 50. Sloan J. Using SAS software to analyze paired comparison data via Coomb's unidimensional unfolding model Proceedings of of the 19th SAS Users' Group International (SUGI) 1994:Dallas, TX. 51. Coombs CH. A theory of data. New York: John Wiley & Sons, Inc.; 1964. 52. Waltz CH, Strickland OL, Lenz ER. Other measurement issues: Triangulation. Measurement in nursing and health research. New York, N.Y.: Springer Publishing, Inc.; 2005. 53. Elkin EB, Kim SH, Casper ES, et al. Desire for information and involvement in treatment decisions: elderly cancer patients' preferences and their physicians' perceptions. J Clin Oncol. 2007;25(33):5275-5280. 54. Deber RB, Kraetschmer N, Urowitz S, et al. Do people want to be autonomous patients? Preferred roles in treatment decision-making in several patient populations. Health Expect. 2007;10(3):248-258. 55. Fischer M, Visser A, Voerman B, et al. Treatment decision making in prostate cancer: patients' participation in complex decisions. Patient Educ Couns. 2006;63(3):308313. 56. Stiggelbout AM, Kiebert GM. A role for the sick role. Patient preferences regarding information and participation in clinical decision-making. CMAJ. 1997;157(4):383389. 57. Bilodeau BA, Degner LF. Information needs, sources of information, and decisional roles in women with breast cancer. Oncol Nurs Forum. 1996;23(4):691-696. 58. Ryan J, Sysko J. The contingency of patient preferences for involvement in health decision making. Health Care Manage Rev. 2007;32(1):30-36. 129 59. Janz NK, Wren PA, Copeland LA, et al. Patient-physician concordance: preferences, perceptions, and factors influencing the breast cancer surgical decision. J Clin Oncol. 2004;22(15):3091-3098. 60. Caldon LJ, Walters SJ, Reed MW. Changing trends in the decision-making preferences of women with early breast cancer. Br J Surg. 2008;95(3):312-318. 61. Fronstin P, Collins SR. The 2nd annual EBRI/Commonwealth Fund Consumerism in Health Care Survey, 2006: early experience with high-deductible and consumerdriven health plans. EBRI Issue Brief. 2006(300):1,5-47. 62. Donaldson C, Lloyd P, Lupton D. Primary health care consumerism amongst elderly Australians. Age Ageing. 1991;20(4):280-286. 63. Beisecker AE. Aging and the desire for information and input in medical decisions: patient consumerism in medical encounters. Gerontologist. 1988;28(3):330-335. 64. Rosenstein AH. Consumerism and health care. Will the traditional patient-physician relationship survive? Postgrad Med. 1986;79(1):13, 16, 18. 130 CHAPTER V The Priority of Information Needs of Older Adults Newly Diagnosed with Myeloma Introduction Providing pertinent information to patients with cancer with which to understand the diagnosis and its consequences and make treatment and self-care decisions is considered an important part of standard care.1 Evaluating which information needs are of highest priority has been studied in breast, prostate, and other cancer patient populations;2 however, cancer patients continue to have unmet information needs, characterized by different information needs and information-seeking behaviors among patients with different types of cancer.3 In a seminal paper, Degner and colleagues4 argued that in an era of scarce health care resources, patient information needs are best prioritized. Prioritization directs the attention of nurses to the most important information needs, enhances the delivery of information that patients need, and provides relevant information to patients at specific periods of their illness. Additionally, prioritization of information needs can make patient encounters more meaningful. As reviewed by Husson and colleagues,5 when information is individualized to fulfill what a patient deems important, the anxiety and depression associated with treatment decision making may be lower. Nurses play a critical role in giving patients the information they need and can potentially help patients assume a more active role in decision making when desired or feel more comfortable when decisions are left to the provider. Conversely, a lack of information can hinder patients’ participation in treatment decision making or increase uncertainty. Patients often report difficulties obtaining the type and amount of information they want or need.6-8 Oncology nurses are poised to provide useful, concise, and relevant information, but 131 they have significant time constraints to provide full information that most cancer patients need.9 By determining the particular types of information that patients view as important, nurses can potentially close the gap between what patients want to know and what nurses believe patients need to know. A new diagnosis of myeloma involves a wide choice of drugs and treatment regimens, including thalidomide, bortezomib, lenalidomide, and liposomal doxorubicin.10, 11 Advances in hematopoietic stem cell transplantation also have increased the treatment options for patients with myeloma.12, 13 Moreover, patients have many information needs, not only in terms of their treatment choices, but in terms of treatment side effects, supportive care, and overall quality of life. Older adults with cancer have various information needs that are oftentimes not met by clinicians, which may be different than the information needs of their younger counterparts.14 Studies of information needs priorities involving older adults revealed a different priority of information needs profile than their younger counterparts.15-18 Since myeloma frequently affects older adults, studying the priority of information needs of these patients could reveal a different priority of information needs than for younger myeloma patients. Purpose The purpose of this study was to examine the priority information needs of a sample of older adults (60 years and older) newly diagnosed with myeloma and to explore whether information needs were influenced by sociodemographic variables such as age, gender, education, relationship status, and income. 132 Methods Design and Sample A cross-sectional survey approach was employed involving administration of an information needs questionnaire (INQ) with 36 paired comparisons during a one-time semistructured interview. The convenience sample consisted of 20 older adults referred through the Seattle Cancer Care Alliance (SCCA) or the Northwestern University Myeloma Program (NUMP) by hematologists/oncologists in the greater Seattle or Chicago areas, respectively. Eligibility criteria included older adults 60 years of age and above who were (a) newly diagnosed with multiple myeloma, (b) able to read and write English, and (c) able to give informed consent. Procedures After obtaining approval from the University of Washington (UW) and Northwestern University (NU) Human Subjects Divisions, older adults newly diagnosed with myeloma were recruited to participate in the study. The researcher made every attempt to recruit from both university- and community-based medical practices in order to recruit diverse study participants. Adults ages 60 and older who had an appointment at SCCA hematology or transplant service and were found to be eligible for the study were recruited by mail using a recruitment flyer. At NUMP, the researcher utilized a direct approach in the recruitment of study participants. A review of clinic schedules at SCCA and NUMP was performed weekly to identify potential study participants. UW-affiliated clinics were also checked weekly for potential study participants. A total of 79 potential participants were recruited by mail at SCCA from October 2009 through July 2010. Fourteen participants responded and participated in the study (a 17.7 % response rate). At NUMP, all six potential participants 133 agreed to participate (a 100% response rate). Informed consent was obtained from all study participants. The Information Needs Questionnaire (INQ)4 was administered by the researcher during a semi-structured interview conducted in designated research-related conference rooms at SCCA and NUMP. These rooms were strictly assigned for research use only and met the Human Subject Division’s standard for patient privacy. If a participant wanted the interview to be conducted at a later time, a one-week period following the appointment was allowed for re-scheduling. Moreover, if a participant wanted the interview to be conducted at his/her home, the researcher conducted the interview at the participant’s home as requested. The participants were informed they could end the interview at any time and refuse to answer specific questions. Instrument The INQ was initially developed by Degner and colleagues,3 applied first in studies of women with breast cancer15, 19 and extended by Davison in her work with men diagnosed with prostate cancer.20, 21 The questionnaire has been found to be valid and reliable in determining the priorities of information needs of patients diagnosed with various types of cancer.4, 15, 17, 19, 20, 22-24 The INQ contains nine categories of information topics each with a statement of definition: (a) prognosis [likelihood of cure], (b) stage of disease [spread and extent of cancer], (c) side effects [possible side effects of treatment], (d) treatment options [treatment available], (e) social activities [impact on work, daily activities, and social life], (f) family risk [hereditary risk], (g) home self-care [health care needs during and following treatment], (h) impact on family [helping family members deal with cancer diagnosis], and 134 (i) sexuality [treatment options and counseling for sexual concerns] (See Table 1). The Thurstone25 scaling method is the traditional analytic approach for the INQ.4 Table 1. The Nine Items of Information Represented in the Information Needs Questionnaire (INQ), Adapted for Myeloma 1 Information about how advanced the disease is and how far it has spread 2 Information about the likelihood of being cured of myeloma 3 Information about how the treatment may affect my ability to carry on my usual social activities 4 Information about how my family and close friends may be affected by myeloma 5 Information about caring for myself at home 6 Information about how the treatment may affect my feelings about my body and sexual attractiveness 7 Information about different types of treatment and the advantages and disadvantages of each treatment 8 Information about whether my children or other members of my family are at risk of getting myeloma 9 Information about unpleasant side effects of treatment Analysis and Instrument Performance Scaling Techniques - Thurstone Scaling Method There are two main types of measurement scales that have been used in cancer research: summated and differential. Summated scales include scales that use a Likert-type scale such as 1 (completely agree) to 12 (completely disagree).26 Studies that use summated scales have demonstrated problems with a “ceiling effect,” where the studied group tends to rate items close to the upper limit of the scale for each category, ranking any information topic as low that may not be socially desirable.4 Ceiling effect can also occur if the study participants have very little variability in the psychological attribute being measured. The ceiling effect has been demonstrated in studies conducted by Davison et al.20 and Degner and colleagues4, 15 when utilizing summated scales. Summated scales have also been criticized for reliability and validity problems due to lack of approximation of equal units and meaningful 135 order, both of which are key aspects of interval-level measurement.27 As suggested by Degner and colleagues,4 when ceiling effects are a common occurrence, an alternative scaling model is needed, so the Thurstone Scale Case V Model - the standard approach among five cases in Thurstone’s model of comparative judgment25 - was used in this study. In 1927, Thurstone28 postulated that each component in a set of stimuli, such as the nine items of the INQ, will possess some attribute (e.g., a particular information item will have a lower or higher value of priority, but the expected level of priority is unknown). Thurstone’s Law of Comparative Judgment would predict that respondents will “prefer one item over other items” (p.60)29 for each of the nine items derived from the INQ. Additionally, the law assumes that the differences in the standard deviation (i.e., preference proportions) of all the participants’ responses to each item in the scale would be equal. The more participants select one item of a pair over the other item, the greater the preference for, or perceived priority of, that item and thus the greater its scale weight. The scale weight was calculated by using the actual percentage of preferred items and was then used to rank the nine items of the INQ. Detailed descriptions of the methods used to execute Thurstone’s paired comparison can be obtained from Sloan and colleagues.29 The INQ was selected for the current study because it has been piloted in several patient samples in cancer settings and shown to capture the information needs that patients consider priorities. These items were checked for simplicity, avoiding confusion among participants, and meeting the requirement of easy interpretability. For Thurstone’s paired comparison scaling to be carried out, it has been suggested to compare a few items.30 The INQ had nine information categories, which was the maximum number of items that would still avoid subject fatigue. With nine items in the INQ, 36 pairings are required to obtain 136 every possible pairing. To avoid selection bias, the Ross (1974)31 matrix of optimal ordering was used to determine the exact same order in which the 36 paired items would be presented to all study participants. This method of ordering ensures maximum time and space distancing among all the possible 36 pairs of information items. For this study, data from the INQ were analyzed using the traditional Thurstone scaling, as entered into the Predictive Analytic SoftWare (PASW) Statistics version 18 (Armonk, NY). Preference proportions were calculated, then translated into standard normal scores (z-scores), representing the participant’s perceived priorities of the information items. A score below zero meant that the number of participants who considered the information item as a priority was less than half of the total number of participants. The information item that had the highest score was prioritized by the majority of the participants. When the zscore is at 0, it meant that the prioritization of that particular information item was ranked by half of the participants. Validity of INQ The validity of the INQ was tested by examining differences in Thurstone scores. Testing of the validity of the INQ is critical to determine whether or not the observed differences between the scale scores for individual items were merely sampling fluctuation caused by chance or a significant difference in terms of level of priority.29 Multiple t-tests were used to test for significant differences between Thurstone scale scores across the respondents. A standard error was calculated from the individual normal deviations that produce the Thurstone scores. A Bonferroni correction to the p-value of each participant was applied to correct for multiple t-tests conducted across all participants’ Thurstone scale scores.29 137 Reliability of INQ The reliability of the INQ was determined by computing the response variability/scalability of the scores and by checking internal model consistency. Response Variability/Scalability of Score. As recommended by the primary authors of the INQ, Gulliksen and Tukey,32 a reliability statistic comparable to the R-square statistic was used to describe the reliability of participants’ individual responses to the information needs questionnaire. Gulliksen and Tukey's statistic measures the extent to which the Thurstone scale scores adequately explains the differences in the individual’s actual values from the expected values, utilizing analysis of variance theory.29 Similar to the R-square statistic, the greater the Gulliksen and Tukey R2 statistic, the greater the scalability of the data.4 Our results indicate that the participants’ individual responses to the nine items in the INQ were moderately reliable as indicated by the Gulliksen and Tukey reliability measure value (R2 = .742) as shown in Table 2. Internal Rater Consistency. The internal rater consistency was established by calculating the circular triads (see below), Kendall’s consistency coefficient (zeta) and Kendall’s coefficient of agreement. Mosteller’s chi-square test of internal consistency33 was used to measure internal model consistency. Mosteller’s chi-square test is a goodness-of-fit test that checks the adequacy of the model fit for Thurstone scaling models (in this study, Thurstone’s Case V model). The assumptions for the Thurstone Case V model in this study are as follows:25, 29, 34 The value of the psychological attribute (i.e., priority for the INQ) has a normal distribution Measurement of only one attribute (i.e., priority) 138 The differences between the expected and actual values (standard deviations) of the psychological attribute on any given paired items of the INQ are equal The Mosteller’s chi-square test and the p-value are used to determine how the model fits the data. The null hypothesis is that the resulting scale values are non-significant, or in other words, that the expected scale values fit the observed data.29 The Mosteller’s chi-square for the present study was 39.39 (p = .075) with 28 degrees of freedom. The null hypothesis is not rejected, indicating that the Case V model fits the data (See Table 2), and therefore it is reasonable to conclude there is demonstrable consistency between the model and the data. Table 2. Gulliksen & Tukey and Mosteller’s Chi-square for INQ for Myeloma Gulliksen & Tukey R-square (% of total Mosteller variance chi-square accounted statistic Mosteller for by scale for internal Degrees of Mosteller N of scores) consistency freedom p-value raters .74175 39.38729 28.00 .07489 20 Circular triads. Internal consistency of an individual’s comparative judgment is assessed by computing the number of circular triads present in the comparisons.29 By calculating circular triads, the consistency of participants in selecting the information category which they considered a priority is established. A participant is said to have a circular triad when a participant is not consistent with his or her comparative judgment. As explained by Edwards (1974),35 when a participant is presented with all three possible pairs of items 1, 2, and 3 from the INQ, the participant must prioritize item 1 over item 2, item 2 139 over item 3 and item 1 over item 3. In this specific example, if the participant prioritizes item 3 over item 1, a circular triad has occurred. In the present study, two of the twenty (10%) participants were always consistent in their choices, with no circular triads, demonstrating complete consistency in their judgment of which information category was more important. Only one participant had 24 circular triads, which could mean that the participant was confused about the items, bored from answering the 36 item questionnaire, or had difficulties in distinguishing one item from another. Overall, the total number of circular triads made by all participants appears to support a moderately strong internal rater consistency among the participants’ comparative judgments. The range of circular triads for this study was 0-24 as shown in Table 3. 140 TABLE 3. Circular Triads and Kendall’s (zeta) Consistency Coefficient Number of circular triads per person Maximum possible n of triads Critical value for n of triads Kendall zeta consistency Degrees coeffiof cient freedom Chi square P value for Kendall zeta Consistency of item rating ______ ______ ______ ______ ______ ______ ______ ______ 12.00 7.00 4.00 .00 11.00 14.00 24.00 9.00 4.00 4.00 7.00 14.00 1.00 7.00 11.00 8.00 12.00 5.00 1.00 .00 30.00 30.00 30.00 30.00 30.00 30.00 30.00 30.00 30.00 30.00 30.00 30.00 30.00 30.00 30.00 30.00 30.00 30.00 30.00 30.00 27.24 27.24 27.24 27.24 27.24 27.24 27.24 27.24 27.24 27.24 27.24 27.24 27.24 27.24 27.24 27.24 27.24 27.24 27.24 27.24 .6000 .7667 .8667 1.0000 .6333 .5333 .2000 .7000 .8667 .8667 .7667 .5333 .9667 .7667 .6333 .7333 .6000 .8333 .9667 1.0000 20.16 20.16 20.16 20.16 20.16 20.16 20.16 20.16 20.16 20.16 20.16 20.16 20.16 20.16 20.16 20.16 20.16 20.16 20.16 20.16 35.36 43.36 48.16 54.56 36.96 32.16 16.16 40.16 48.16 48.16 43.36 32.16 52.96 43.36 36.96 41.76 35.36 46.56 52.96 54.56 .9807 .9980 .9996 .9999 .9874 .9562 .2843 .9949 .9996 .9996 .9980 .9562 .9999 .9980 .9874 .9968 .9807 .9993 .9999 .9999 .00 .00 .00 .00 .00 .00 .00 .00 .00 .00 .00 .00 .00 .00 .00 .00 .00 .00 .00 .00 Kendall’s zeta coefficient (range 0 to 1) was used to test the null hypothesis that the presence of triads was due to chance or indicative of inconsistent logic. The closer the coefficient is to 1, the more consistent the participants are in their comparative judgments of the preferred items. In this study the test for Kendall’s zeta coefficient showed that the majority of the participants (90%) were consistent with their comparative judgments of the preferred items. According to Sloan and colleagues,29 a limitation of this statistic is that it is quite insensitive, so a large number of circular triads (i.e. ≥27 circular triads) would have to be present for the responses to be identified as inconsistent. The possibility of such an 141 occurrence was seen in only 1 (5%) out of the twenty participants; this participant had 24 circular triads and was nearing the critical value of 27 circular triads. Kendall’s coefficient of agreement was used to determine the extent of agreement among participants with reference to their comparative judgment of the 36 paired comparisons found in the INQ. When the coefficient is higher (0 to 1), it means there is higher agreement among participants regarding their overall priorities for the nine items in the INQ. Sloan and colleagues29 emphasized that this test has a broad null hypothesis (i.e., departure from random preference rating), frequently resulting in a statistically significant test even though the level of agreement among participants could still be low. The present study showed that the agreement among myeloma patients in their paired comparative judgment is low but it is still statistically significant (W=.25; p<0.001) as seen in Table 4. Table 4. Kendall’s Coefficient of Agreement Number of raters Number of items Kendall Coefficient of agreement Chi-square Degrees of freedom p-value indicates agreement among raters _______ _______ _______ ________ _______ ________ 20 9 .25526 236.22222 42.22 .001 ___________________________________________________________________________ Association of Sociodemographic Variables with Information Priorities Differences in information priorities across dichotomous categories of age (>70 years versus ≤70 years), education (less than 4 years of college versus greater than 4 years of college), relationship status (single versus partnered), income (<$55,000 versus ≥$55,000), and employment status (retired versus non-retired) were compared using Student’s t-tests. 142 Given the results of the bivariate analysis and the small sample size, multivariate analyses are unwarranted. Results Table 5 presents the sociodemographic characteristics of the 20 study participants. Participants’ mean age was 67.5 years; median 62.5 years. The majority of participants in this sample were Caucasian (90%), female (60%), partnered (60%), retired (65%) and had at least a 2-year college education (75%); half the sample reported an annual household income >$55,000 Table 5. Sociodemographic Characteristics of the Sample Variable Age 60-70 71-82 Gender Male Female Race Caucasian Asian American Indian/Native Alaskan Employment Status Full time Working on medical leave Not working Retired Student N % 14 6 70 30 8 12 40 60 18 1 1 90 5 5 2 2 2 13 1 10 10 10 65 5 143 Table 5 continued Variable Personal Relationship Status Single Married or partnered Divorced Widowed Highest Level of Education 9th – 12th grade 2 year college 4 year college Graduate degree Annual Household Income $18,000 or less $18,000 to $35,000 $35,001 to $55,000 $55,001 to $85,000 $85,001 and above N % 2 12 5 1 10 60 25 5 5 2 10 3 25 10 50 15 3 2 5 5 5 15 10 25 25 25 Preference Frequencies The number of participants who preferred one item over another for all the comparisons is shown in Table 6. Using the preference frequencies, the preference proportion was obtained by dividing the preference percentage by the total number of respondents. The more participants that select one item of a pair over the other item, the greater the preference for, or perceived priority of, that item, and thus the greater its scale weight. The scale weight was calculated by using the actual percentage of preferred items and was then used to rank the nine items of the INQ. 144 Table 6. Frequencies of Participants’ Preference for 36 Paired Items in the INQ Comparison Presented Item Preference Item 1 versus Item 2 Second item preferred First item preferred Total Response Yes 13 0 13 Total Responses 13 7 20 10 0 10 10 10 20 .00 0 1.00 8 Total 8 12 12 0 8 12 20 .00 0 1.00 11 Total 11 9 9 0 11 9 20 .00 0 1.00 2 Total 2 18 18 0 2 18 20 .00 0 1.00 18 Total 18 2 2 0 18 2 20 No 0 7 7 I31 Item 1 versus Item 3 Second item preferred First item preferred Total 0 10 10 I41 Comparison of Item 1 versus Item 4 Total Second item preferred First item preferred I51 Comparison of Item 5 versus Item 1 Total Second item preferred First item preferred I61 Comparison of Item 6 versus Item 1 Total Second item preferred First item preferred I71 Comparison of Item 7 versus Item 1 Total Second item preferred First item preferred 145 Table 6 continued Comparison Presented Item Preference Response Yes Total Responses .00 0 1.00 8 Total 8 12 12 0 8 12 20 .00 0 1.00 11 Total 11 9 9 0 11 9 20 .00 0 1.00 8 Total 8 12 12 0 8 12 20 .00 0 1.00 7 Total 7 13 13 0 7 13 20 .00 0 1.00 8 Total 8 12 12 0 8 12 20 .00 0 1.00 16 Total 16 4 4 0 16 4 20 No I81 Comparison of Item 8 versus Item 1 Total Second item preferred First item preferred I91 Comparison of Item 9 versus Item 1 Total Second item preferred First item preferred I23 Comparison of Item 2 versus Item 3 Total Second item preferred First item preferred I42 Comparison of Item 4 versus Item 2 Total Second item preferred First item preferred I25 Comparison of Item 2 versus Item 5 Total Second item preferred First item preferred I62 Comparison of Item 6 versus Item 2 Total Second item preferred First item preferred 146 Table 6 continued Comparison Presented Item Preference Response No Yes Total Responses I27 Comparison of Item 2 versus Item 7 Total Second item preferred First item preferred .00 0 1.00 11 Total 11 9 9 0 11 9 20 .00 0 1.00 13 Total 13 7 7 0 13 7 20 .00 0 1.00 6 Total 6 14 14 0 6 14 20 .00 0 1.00 6 Total 6 14 14 0 6 14 20 .00 0 1.00 5 Total 5 15 15 0 5 15 20 .00 0 1.00 3 Total 3 17 17 0 3 17 20 I82 Comparison of Item 8 versus Item 2 Total Second item preferred First item preferred I29 Comparison of Item 2 versus Item 9 Total Second item preferred First item preferred I34 Comparison of Item 3 versus Item 4 Total Second item preferred First item preferred I53 Comparison of Item 5 versus Item 3 Total Second item preferred First item preferred I36 Comparison of Item 3 versus Item 6 Total Second item preferred First item preferred 147 Table 6 continued Comparison Presented Item Preference Response Yes Total Responses .00 0 1.00 0 Total 0 20 20 0 0 20 20 .00 0 1.00 11 Total 11 9 9 0 11 9 20 .00 0 1.00 6 Total 6 14 14 0 6 14 20 .00 0 1.00 14 Total 14 6 6 0 14 6 20 .00 0 1.00 18 Total 18 2 2 0 18 2 20 .00 0 1.00 19 Total 19 1 1 0 19 1 20 No I73 Comparison of Item 7 versus Item 3 Total Second item preferred First item preferred I38 Comparison of Item 3 versus Item 8 Total Second item preferred First item preferred I39 Comparison of Item 3 versus Item 9 Total Second item preferred First item preferred I45 Comparison of Item 4 versus Item 5 Total Second item preferred First item preferred I64 Comparison of Item 6 versus Item 4 Total Second item preferred First item preferred I47 Comparison of Item 4 versus Item 7 Total Second item preferred First item preferred 148 Table 6 continued Comparison Presented Item Preference Response No Yes Total Responses I48 Comparison of Item 4 versus Item 8 Total Second item preferred First item preferred .00 0 1.00 12 Total 12 8 8 0 12 8 20 .00 0 1.00 8 Total 8 12 12 0 8 12 20 .00 0 1.00 0 Total 0 20 20 0 0 20 20 .00 0 1.00 16 Total 16 4 4 0 16 4 20 .00 0 1.00 10 Total 10 10 10 0 10 10 20 .00 0 1.00 10 Total 10 10 10 0 10 10 20 I94 Comparison of Item 9 versus Item 4 Total Second item preferred First item preferred I56 Comparison of Item 5 versus Item 6 Total Second item preferred First item preferred I57 Comparison of Item 5 versus Item 7 Total Second item preferred First item preferred I85 Comparison of Item 8 versus Item 5 Total Second item preferred First item preferred I59 Comparison of Item 5 versus Item 9 Total Second item preferred First item preferred 149 Table 6 continued Comparison Presented Item Preference Response Yes Total Responses .00 0 1.00 1 Total 1 19 19 0 1 19 20 .00 0 1.00 17 Total 17 3 3 0 17 3 20 .00 0 1.00 2 Total 2 18 18 0 2 18 20 .00 0 1.00 16 Total 16 4 4 0 16 4 20 .00 0 1.00 4 Total 4 16 16 0 4 16 20 .00 0 1.00 3 Total 3 17 17 0 3 17 20 No I76 Comparison of Item 7 versus Item 6 Total Second item preferred First item preferred I68 Comparison of Item 6 versus Item 8 Total Second item preferred First item preferred I96 Comparison of Item 9 versus Item 6 Total Second item preferred First item preferred I87 Comparison of Item 8 versus Item 7 Total Second item preferred First item preferred I79 Comparison of Item 7 versus Item 9 Total Second item preferred First item preferred I98 Comparison of Item 9 versus Item 8 Total Second item preferred First item preferred Priorities of Information Needs (Rank Ordering of Nine Information Items) 150 The Thurstone scale analysis for 20 participants newly diagnosed with myeloma indicated that the top three priority information needs were related to: the “different types of treatments” and corresponding advantages/disadvantages, the “likelihood of cure” and “caring for myself at home.” The item about the impact of treatment on “feelings about my body and sexual attractiveness” was ranked in last place (See Figure 1). When asked if there were any other items of information that were important but had not been included in the INQ, two individuals in this study gave an affirmative response. These comments by two participants included information needs related to stem cell transplantation (What is the length of hospital stay?) and overall survival (How long will I live with myeloma?). 151 Figure 1. Priority of information needs by rank. The items on the left are ranked 1-9 starting from the top to bottom. The items on the right are the Case V scores. Sociodemographic Variables Analysis of information needs by subgroup revealed no statistically significant differences across dichotomous categories of age (>70 years versus ≤70 years), education (<4 years of college versus ≥years of college), partner status (single versus partnered), income (<$55,000 versus ≥$55,000), or employment status (retired versus non-retired). Discussion These results suggest that older adults newly diagnosed with myeloma have a profile of information needs priorities that clinicians can use to initiate information exchange. The profiles suggest that older adults newly diagnosed with myeloma want to discuss the 152 treatment options available to them as well as how these options may impact myeloma outcomes. Since the outcome of myeloma is not heavily influenced by the stage of disease at the time of diagnosis compared to other malignancies, this could explain why the study participants ranked disease stage as the fifth priority out of nine priorities. In contrast to younger cancer patients, who have been shown in several studies to have prognosis, disease stage and treatment options as their top three priorities,15-17 this study demonstrated that the priority of information needs for older patients was information about treatment options, likelihood of cure and self-care. Older adults value independence;36 hence, it was not surprising to find that self-care was one of the top three priority information needs. This finding is supported by another study, which found that older breast cancer patients (65 and above years of age) ranked self-care information more important than did younger patients.19 Another study involving older participants diagnosed with esophageal cancer in Sweden (58-86 years old, with a mean of 69 years of age) also found treatment and self-care in the top three priorities of information needs, and sexual attractiveness was ranked as the last priority.37 In contrast to the current study, another study that involved younger breast cancer patients in Malaysia (32-60 years of age, with a mean of 45.09 years of age) found that sexual attractiveness was ranked as the second top priority information need by the participants.38 The rankings in this study must be interpreted with caution, because a single assessment of information needs with a small sample size may be unreliable. As suggested by Butow and others, a periodic assessment of an individual patient’s information needs is imperative, given the changing dynamics of patient’s information needs priorities.23, 39, 40 153 Because of the ability of the Thurstone scaling method to circumvent the ceiling effect commonly seen in Likert-type summated scale, the utility of INQ in prioritizing the information needs of large group of patient subgroups is promising. In this study, the Thurstone scaling successfully produced a profile of information needs priorities in older adults diagnosed with myeloma. The Thurstone’s paired comparison scaling has strong psychometric properties as evidenced by statistically significant R-square statistics and Kendall’s coefficient of agreement. These results validate the psychometric properties of INQ instrument, as reported in other studies.15-20, 38, 41, 42 Several study limitations must be acknowledged. The generalizability of study findings is limited to Caucasian men and women who are relatively highly educated and are receiving care at a university-based comprehensive cancer center. The low response rate (17.7%) is a potential source of response bias. One could argue that patients who did not respond to the mailed flyer may be sicker than those who positively responded, and potentially have different profiles of information needs. Additionally, the sample size was small, which prevents the findings from being generalized to larger groups and also limits the comparison of differences of information needs by other variables. Moreover, since this is a cross-sectional study, the findings may not be applicable to myeloma patients who are beyond the first 6 months from diagnosis and these results do not explain any aspect of changes in information priorities over time. Despite these limitations, the study is still of value because it’s the first one to identify information needs in myeloma patients and offers additional hypotheses that need to be explored in older cancer patients. A longitudinal approach in a future study should be able to document any changes in the priorities of 154 information needs at different periods of the disease trajectory (i.e., newly diagnosed, first relapse, relapsed/refractory stages). Implications for Practice and Research The findings of this study have implications for nurses who are caring for older adult patients newly diagnosed with myeloma. The relative priority of information needs presented in this study could serve as a guide to elicit the individual information needs of future myeloma patients. For example, Luker and colleagues have suggested that a profile of patient information needs can be used as a clinical reference tool to create a structured approach to information giving.22 By profiling information needs in advance, nurses may better communicate myeloma-related information to their patients, thereby improving practice and outcomes. A computerized version of INQ has already been tested and can efficiently produce a patient’s priority of information needs immediately.41, 43 The use of this technology, coupled with adequate knowledge and communication skills of the nurse, could potentially offer some innovative solutions to the persistent problem of shrinking time for patient education. Studies utilizing the computerized version of INQ to assess information priorities in older adult populations are still needed. The priorities of information needs elicited from this study should serve as a guide in prioritizing information exchange with patient. In terms of treatment and prognosis, the physician will be the best clinician to discuss the probability of survival in quantitative terms to help patients make an informed decision. The physician has the full knowledge of potential risks and benefits of therapy and should address this issue first with the patient. Nurses can provide important supplemental information to patients in terms of how the therapy will work and what side effects to expect. Self-care management strategies of side 155 effects can also be provided by the nurse. Of all the patient’s caregivers, the nurse is often in contact with patient to provide additional information related to possible treatments, prognosis, and self-care. Where information is scant, a nurse can always consult with the multidisciplinary team members such as the clinical psychologist and social worker in order to provide the information that the patient needs. Additionally, the nurse has the ability to share important patient information with physicians, psychologists, and social workers including information that relate to self-care, family, and social life. Although the profile of information needs provides a quick summary of the types of information a patient needs, it is still critical to perform an individual assessment of information needs for each patient. The hierarchies of information needs identified in this study are based on grouped data that are meant to provide general guides for the provision of information in clinical practice. As most researchers suggest, individualized assessment of information needs at various time points is imperative, since a patient’s information needs will usually shift over time. Longitudinal studies are needed to help clinicians identify the appropriate frequency of assessment of patient information needs. Conclusion The study findings demonstrate that older adults newly diagnosed with myeloma are able to prioritize information needs. Oncology nurses and other health care professionals responsible for providing patient education should assess patient’s information needs, and could use the top three priorities relating to treatment, prognosis, and self-care reported in this study as a starting board to elicit the patient’s information needs and share those information needs with other members of the health care team. 156 References 1. Jacobson JO, Polovich M, McNiff KK, et al. American Society of Clinical Oncology/Oncology Nursing Society chemotherapy administration safety standards. Oncol Nurs Forum. 2009;36(6):651-658. 2. Gaston CM, Mitchell G. Information giving and decision-making in patients with advanced cancer: a systematic review. Soc Sci Med. 2005;61(10):2252-2264. 3. Nagler RH, Gray SW, Romantan A, et al. Differences in information seeking among breast, prostate, and colorectal cancer patients: Results from a population-based survey. Patient Educ Couns. 2010;81 Suppl:S54-62. 4. Degner LF, Davison BJ, Sloan JA, et al. Development of a scale to measure information needs in cancer care. J Nurs Meas. 1998;6(2):137-153. 5. Husson O, Mols F, van de Poll-Franse LV. The relation between information provision and health-related quality of life, anxiety and depression among cancer survivors: a systematic review. Ann Oncol. 2010. Epub ahead of print. 6. Walsh MC, Trentham-Dietz A, Schroepfer TA, et al. Cancer information sources used by patients to inform and influence treatment decisions. J Health Commun. 2010;15(4):445-463. 7. Mistry A, Wilson S, Priestman T, et al. How do the information needs of cancer patients differ at different stages of the cancer journey? A cross-sectional survey. JRSM Short Rep. 2010;1(4):30. 8. Lewis N, Gray SW, Freres DR, et al. Examining cross-source engagement with cancer-related information and its impact on doctor-patient relations. Health Commun. 2009;24(8):723-734. 157 9. Ousley AL, Swarz JA, Milliken EL, et al. Cancer education and effective dissemination: information access is not enough. J Cancer Educ. 2010;25(2):196-205. 10. Tariman JD, Faiman B. Multiple Myeloma. In: Yarbro CH, Wujcik D, Gobel BH, eds. Cancer Nursing Principles and Practice. 7th ed. Sadbury, MA: Jones and Bartlett Publishers; 2011:1513-1545. 11. Rome S. Patient teaching. In: Tariman JD, ed. Multiple myeloma: a textbook for nurses. Pittsburgh, PA: Oncology Nursing Society; 2010:195-229. 12. Palumbo A, Gay F, Falco P, et al. Bortezomib as induction before autologous transplantation, followed by lenalidomide as consolidation-maintenance in untreated multiple myeloma patients. J Clin Oncol. 2010;28(5):800-807. 13. Minnema MC, van der Spek E, van de Donk NW, et al. New developments in the treatment of patients with multiple myeloma. Neth J Med. 2010;68(1):24-32. 14. Chouliara Z, Kearney N, Stott D, et al. Perceptions of older people with cancer of information, decision making and treatment: a systematic review of selected literature. Ann Oncol. 2004;15(11):1596-1602. 15. Degner LF, Kristjanson LJ, Bowman D, et al. Information needs and decisional preferences in women with breast cancer. JAMA. 1997;277(18):1485-1492. 16. Wallberg B, Michelson H, Nystedt M, et al. Information needs and preferences for participation in treatment decisions among Swedish breast cancer patients. Acta Oncol. 2000;39(4):467-476. 17. Davison BJ, Gleave ME, Goldenberg SL, et al. Assessing information and decision preferences of men with prostate cancer and their partners. Cancer Nurs. 2002;25(1):42-49. 158 18. Luker KA, Beaver K, Leinster SJ, et al. The information needs of women newly diagnosed with breast cancer. J Adv Nurs. 1995;22(1):134-141. 19. Bilodeau BA, Degner LF. Information needs, sources of information, and decisional roles in women with breast cancer. Oncol Nurs Forum. 1996;23(4):691-696. 20. Davison BJ, Degner LF, Morgan TR. Information and decision-making preferences of men with prostate cancer. Oncol Nurs Forum. 1995;22(9):1401-1408. 21. Davison BJ, Kirk P, Degner LF, et al. Information and patient participation in screening for prostate cancer. Patient Educ Couns. 1999;37(3):255-263. 22. Luker KA, Beaver K, Leinster SJ, et al. The information needs of women newly diagnosed with breast cancer. J Adv Nurs. 1995;22(1):134-141. 23. Luker KA, Beaver K, Leinster SJ, et al. Information needs and sources of information for women with breast cancer: a follow-up study. J Adv Nurs. 1996;23(3):487-495. 24. Beaver K, Bogg J, Luker KA. Decision-making role preferences and information needs: a comparison of colorectal and breast cancer. Health Expect. 1999;2(4):266276. 25. Thurstone LL. Psychophysical analysis. In: Maranell GM, ed. Scaling: A sourcebook for behavioral scientists. Chicago, IL: Aldine; 1974. 26. Waltz CH, Strickland OL, Lenz ER. An introduction to measurement. Measurement in nursing and health research. New York, NY: Springer Publishing Company, Inc.; 2005:3-21. 27. Waltz CH, Strickland OL, Lenz ER. Measurement theories and frameworks. Measurement in nursing and health research. New York, NY: Springer Publishing Company, Inc.; 2005:42-77. 159 28. Thurstone LL. A law of comparative judgement. Psychological Review. 1927;34:273286. 29. Sloan JA, Doig W, Yeung A. A manual to carry out Thurstone scaling and related analytic procedures Manitoba Nursing Research Institute Technical Report #11: University of Manitoba, Canada; 1994. 30. Green B. Paired comparison scaling. In: Maranell GM, ed. Scaling: A sourcebook for behavioral scientists. New Brunswick, N. J.: Aldine Transaction; 1974:93-97. 31. Ross RT. Optimal orders in the method of paired comparisons. In: Maranell GM, ed. Scaling: A sourcebook for behavioral scientists. Chicago, IL: Aldine; 1974:106-109. 32. Bechtel GG. The analysis of variance and pairwise scaling. Psychometrika. 1967;32(1):47-65. 33. Mosteller F. Remarks of the method of paired comparison: a test of significance for paired comparisons when equal standard deviation and equal correlations are assumed. Psychometrika. 1951;41:42-47. 34. Thurstone LL. A law of comparative judgment. In: Maranell GM, ed. Scaling: A sourcebook for behavioral scientists. New Brunswick, N. J.: Aldine Transaction; 1974:81-92. 35. Edwards AL. Circular triads, the coefficient, and the coefficient of agreement. In: Maranell GM, ed. Sacaling: A sourcebook for behavioral scientists. New Brunswick, N. J.: Aldine Transaction; 1974:98-105. 36. Martin VC, Roberto KA. Assessing the stability of values and health care preferences of older adults: A long-term comparison. J Gerontol Nurs. 2006;32(11):23-33. 160 37. Andreassen S, Randers I, Naslund E, et al. Information needs following a diagnosis of oesophageal cancer; self-perceived information needs of patients and family members compared with the perceptions of healthcare professionals: a pilot study. Eur J Cancer Care (Engl). 2007;16(3):277-285. 38. Gopal RL, Beaver K, Barnett T, et al. A comparison of the information needs of women newly diagnosed with breast cancer in Malaysia and the United kingdom. Cancer Nurs. 2005;28(2):132-140. 39. Butow PN, Maclean M, Dunn SM, et al. The dynamics of change: cancer patients' preferences for information, involvement and support. Ann Oncol. 1997;8(9):857863. 40. Vogel BA, Bengel J, Helmes AW. Information and decision making: Patients' needs and experiences in the course of breast cancer treatment. Patient Educ Couns. 2008;71(1):79-85. 41. Davison BJ, Goldenberg SL, Wiens KP, et al. Comparing a generic and individualized information decision support intervention for men newly diagnosed with localized prostate cancer. Cancer Nurs. 2007;30(5):E7-E15. 42. Beaver K, Booth K. Information needs and decision-making preferences: comparing findings for gynaecological, breast and colorectal cancer. Eur J Oncol Nurs. 2007;11(5):409-416. 43. Davison BJ, Goldenberg SL, Gleave ME, et al. Provision of individualized information to men and their partners to facilitate treatment decision making in prostate cancer. Oncol Nurs Forum. 2003;30(1):107-114. 161 Appendix A The Control Preferences Cards 162 163 1 CURRICULUM VITAE Updated March 6, 2011 JOSEPH D. TARIMAN, PhD, APRN, BC EDUCATION Ph.D. in Nursing Science 2011 University of Washington Seattle, WA Post-MSN NP Certificate 2001 University of Miami Coral Gables, FL Master of Arts in Nursing 1993 Cebu Doctors’ University Philippines Bachelor of Science in Nursing 1991 University of the Visayas Philippines PROFESSIONAL LICENSES AND CERTIFICATIONS Current Licenses and Certifications: Registered Nurse IL Dept. of Health RN041324045, expiration 05/31/2012 Advanced Registered Nurse Practitioner IL Dept. of Health APN209004124, expiration 05/31/2012 IL Controlled Substance License-APN, Active, expiration 5/31/2012 Adult Health Nurse Practitioner American Nurses Credentialing Center, #0359713-21 valid until 12/31/2011 Past Licenses and Certification: Registered Nurse 2 WA Dept. of Health RN00163141, expiration 12/05/2010 Advanced Registered Nurse Practitioner WA Dept. of Health AP30007274, expiration 12/05/2010 Oncology Certified Nurse® Oncology Nursing Certification Corporation (2002-2010) PROFESSIONAL EXPERIENCE: ACADEMIC 2009 – present Predoctoral Fellow, National Research Service Award 1F31NR011124-01A1 2006 – 2009 Predoctoral Research Fellow, Biobehavioral Nursing Research Program (NR007106-NIH/NINR) University of Washington Seattle, WA 1992- 1995 Clinical Instructor III School of Nursing Cebu Doctors’ University, Philippines PROFESSIONAL EXPERIENCE: CLINICAL University/Academic Medical Center 2010 – present Advanced Practice Nurse Northwestern University Multiple Myeloma Program Chicago, IL 2006 – 2009 Clinical Research Resident ESRA-C Clinical Trial University of Washington/Seattle Cancer Care Alliance 2002 – 2006 Certified Nurse Practitioner Northwestern University Medical Faculty Foundation Multiple Myeloma Program Chicago, IL 3 2001- 2002 Nurse Manager Hematology-Oncology/ Bone Marrow Transplant Unit University of Chicago Hospitals Chicago, IL 2001 – 2004 Staff Nurse, Per Diem Louis Weiss Memorial Hospital (UC of Hospital Affiliates) Medical-Surgical Oncology Unit Chicago, IL 2000 – 2001 Staff Nurse III University of Miami Hospitals and Clinics-Sylvester Comprehensive Canter Center Hem-Oncology In-patient Unit Miami, FL Non-Academic Centers 2006 – 2009 Staff RN, Per Diem Hematology-Oncology Unit Evergreen Medical Center Kirkland, WA 1999 – 2000 Assistant Director of Nursing Star Multi Care Services, Inc. Hollywood, FL 1998 – 1999 Hi-tech IV/Chemotherapy Case Manager Star Multi Care Services, Inc. Hollywood, FL 1997- 1998 Director of Staff Development and Training Star Multi Care Services D/B/A American Health Care Services Miami Lakes, FL 4 1996 – 1997 Hi-Tech Field Supervisor Home Health Star Multi Care Services D/B/A American Health Care Services Miami Lakes, FL 1995 -1996 Hi-Tech Field RN Home Health Total Care Nursing Services Miami, FL PUBLICATIONS * indicates data based work # indicates work as contributing writer, Advance for NP ≈ indicates invited work ∞ indicates work as contributing editor, ONS Connect (Formerly ONS News) Book 1. ≈Tariman, J. D. (2010). Multiple Myeloma: A Textbook for Nurses. Pittsburgh, PA: Oncology Nursing Society Publishing Division. Book chapters: 1. ≈Tariman, J. D. (2010). Chapter 1: Introduction. In. J. D. Tariman. Multiple Myeloma: A Textbook for Nurses, Pittsburgh, PA: ONS Publishing Division. Pp. 1-7. 2. Tariman, J. D. (2005). Chapter 14: On the horizons: Future considérations. In. J. D. Tariman. Multiple Myeloma: A Textbook for Nurses, Pittsburgh, PA: ONS Publishing Division. Pp. 267-277. 3. Tariman, J. D. & Faiman, B (2011). Chapter 61: Multiple Myeloma. In. C.H. Yarbro, M.H. Frogge, & M. Goodman. Cancer Nursing Principles and Practice, 7th ed. Sudbury, MA: Jones and Bartlett Publishers. Pp. 1513-1545. 4. ≈ Tariman, J. D. (2005). Chapter 59: Multiple Myeloma. In. C.H. Yarbro, M.H. Frogge, & M. Goodman. Cancer Nursing Principles and Practice, 6th ed. Sudbury, MA: Jones and Bartlett Publishers. Pp. 1460-1489. 5 Publications – Peer Reviewed: 1. Tariman, J. D., Berry, D. L., Halpenny, B., Wolpin, S., Schepp, K., (2011). Validation and testing of the Acceptability E-scale for web-based patient reported outcomes in cancer care. Journal of Applied Nursing Research, 24(1), 53-58. 2. Tariman, J. D., Berry, D. L., Cochrane, B., Doorenbos, A., Schepp, K. (2010). Preferred and actual level of participation during health care decision-making in persons with cancer: A systematic review. Annals of Oncology, 21(6), 1145-1151. 3. Rehwaldt, M., Wickham., R, Purl, S., Tariman, J., Blendowski, C., Shott, S., et al. (2009). Self-care strategies to cope with taste changes after chemotherapy. Oncology Nursing Forum, 36(2), E47-56. 4. Colson, K., Doss, D.S., Swift, R., & Tariman, J. (2008). Expanding role of bortezomib in multiple myeloma: Nursing implications. Cancer Nursing, 31(3), 239249. 5. Tariman, J. D., Love, G., McCullagh, E. J., Sandifer, S., IMF Leadership Board. (2008). Peripheral neuropathy associated with novel therapies in patients with multiple myeloma: Consensus statement of the IMF Nurse Leadership Board. Clinical Journal of Oncology Nursing, 12(3, Suppl), 29-35. 6. Bertolotti, P., Bilotti, E., Colson, K., Curran, K., Doss, D., Faiman, B., the IMF Nurse Leadership Board. (2008). Management of side effects of novel therapies for multiple myeloma. Clinical Journal of Oncology Nursing, 12(3, Suppl), 9-12. 7. Faiman, B., Bilotti, E., Mangan, P.A., Rogers, K., & the IMF Nurse Leadership Board. (2008). Steroid-associated side effects in patients with multiple myeloma: Consensus statement of the IMF Nurse Leadership Board. Clinical Journal of Oncology Nursing, 12(3, Suppl), 53-62. 8. Miceli, T., Colson, K., Gavino, M., Lilleby, K., & the IMF Nurse Leadership Board. (2008). Myelosuppression associated with novel therapies in patients with multiple myeloma: Consensus statement of the IMF Nurse Leadership Board. Clinical Journal of Oncology Nursing, 12(3, Suppl), 13-19. 9. Rome, S., Doss, D.S., Miller, K., Westphal, J., & the IMF Nurse Leadership Board. (2008). Thromboembolic events associated with novel therapies in patients with multiple myeloma: Consensus statement of the IMF Nurse Leadership Board. Clinical Journal of Oncology Nursing, 12(3, Suppl), 21-27. 10. Smith, L.C., Bertolotti, P., Curran, K., Jenkins, B., & the IMF Nurse Leadership Board. (2008). Gastrointestinal side effects associated with novel therapies in patients with multiple myeloma: Consensus statement of the IMF Nurse Leadership Board. Clinical Journal of Oncology Nursing, 12(3, Suppl), 37-51. 11. ≈ Tariman, J. D. (2007). Lenalidomide: A promising novel agent for patients with relapsed and/or refractory multiple myeloma. Clinical Journal of Oncology Nursing, 11, 569-574. 12. Rodriguez, A., Tariman, J. D., Enecio, T., Estrella, M. E. (2007). The role of high dose chemotherapy supported by hematopoietic stem cell transplantation in patients with MM: Implications for nursing. Clinical Journal of Oncology Nursing, 11, 579589. 6 13. ≈ Tariman, J. D. (2007). Current therapies for multiple myeloma. Journal of Infusion Nursing, 30(2), 113-118. 14. Chanan-Khan, AA., Kaufman, JL, Mehta, J., Richardson, P.G., Miller, K.C., Lonial, S., Munshi, N. C., Schlossman, R. Tariman, J., et al., (2007). Activity and safety of bortezomib in multiple myeloma patients with advanced renal failure: A multicenter retrospective study. Blood, 109(6), 2604-2606. 15. Tariman, J. D. & Estrella, E. (2005). The changing treatment paradigm in patients with newly diagnosed multiple myeloma: Implications to nursing practice. Oncology Nursing Forum, 31, E127-E138. 16. ≈Colson, K., Doss, D. S., Swift, R., Tariman, J., & Thomas, T. E. (2004). Bortezomib, a newly approved proteasome inhibitor for the treatment of multiple myeloma: Nursing implications. Clinical Journal of Oncology Nursing, 8, 473-480. 17. ≈ Tariman, J. D. (2004). Clinical applications of magnetic resonance imaging in patients with multiple myeloma. Clinical Journal of Oncology Nursing, 8, 317-320. 18. Tariman, J. D. (2003). Understanding novel therapeutic agents for multiple myeloma. Clinical Journal of Oncology Nursing, 7, 521-528. 19. Tariman, J. D. & Lemoine, C (2003). Bortezomib. Clinical Journal of Oncology Nursing, 7, 687-689. 20. Tariman, J. D. (2003). Thalidomide: Current therapeutic uses and management of its toxicities. Clinical Journal of Oncology Nursing, 7, 143-147. Published Abstracts – Peer Reviewed: 1. Heuck, C., Mehta, J., Tariman, J., Pulliam, N., Yu, Y., Bhagat, T., et al. (2010). Epigenomic profiling of multiple myeloma shows widespread stage specific alterations in DNA methylation that occur early during myelomagenesis [Abstract #784]. Available at: http://ash.confex.com/ash/2010/webprogram/Paper33425.html 2. Tariman, J. D., Berry, D. L., Cochrane, B., Doorenbos, A., & Schepp, K. (2010). Treatment decision-making in older adults newly diagnosed with myeloma: A Preliminary analysis. Communicating Nursing Research (CD-ROM). Poster presentation at WIN Conference 2010. 3. Tariman, J. D. (2009). Preferred and actual level of participation during treatment decisions in persons with cancer: A systematic review. Communicating Nursing Research (CD-ROM). Poster presentation at WIN Conference 2009. 4. Tariman, J. D. (2009). A proposed theoretical model of decision making participation, decisional conflict, regret, satisfaction, and QOL in adults with cancer. Communicating Nursing Research (CD-ROM). Poster presentation at WIN Conference 2009. 5. ≈Moran, D., Bertolotti, P., Bilotti, E., Colson, K., Curran, K., Doss, D., Faiman, B., Tariman, J. D., et al. (2009). Long-term care guidelines for myeloma patients [Abstract # 30]. XII Int’l Myeloma Workshop- Washington, DC. February 27, 2009. 6. Tariman, J. D. (2008). Decision-making in oncology settings: A concept analysis. Communicating Nursing Research, 41, 336. 7. Tariman, J.D., Berry, D.L., Halpenny, B., & Wolpin, S. (2008). Validation and testing of the Acceptability E-scale. Communicating Nursing Research, 41, 372. 7 8. ≈Bertolotti, P., Bilotti, E., Colson, K., Curran, K., Doss, D., Faiman, B., Tariman, J. D., et al. (2007). Nursing guidelines for enhanced patient care [Abstract # PO-1102]. Haematologica, 92(6Suppl 2), 211. 9. * Tariman, J. D., Paice, J., Mehta, J., Duffey, S. & Singhal, S. (2007). Quality of life (QOL) in older adults undergoing autologous stem cell transplant (ASCT) [Abstract No. 101]. Oncology Nursing Forum, 34(1), 207. 10. * Rehwaldt, M., Purl, S., Tariman, J. D., Blendowski, C., Lappe, M., Shott, S., & Wickman, R. (2006). A study of taste change strategies [Abstract No. 256]. Oncology Nursing Forum, 33(2), 467. 11. * Gidron, A., Tariman, J., Shabbir, M., Mehta, J., Singhal, S. (2005). High plasma cell labeling index is an adverse prognostic factor in bortezomib-treated patients with relapsed myeloma [Abstract No. 3471]. Blood, 106(11), 968A-969A. 12. * Kut, V., Mehta, J., Tariman, J., Olsson, A., Singhal, S. (2004). Osteonecrosis of the jaw in myeloma patients receiving pamidronate or zoledronate [Abstract No. 4933]. Blood, 104, 315b. 13. * Singhal, S., Tariman, J., Smith, K., Riley, M.B., Mistina, J., Mehta, J. (2003). Bortezomib is effective therapy for relapsed multiple myeloma [Abstract No. 5289]. Blood, 102(11), 390B-391B. 14. * Singhal, S., Goolsby, C., Taylor, R., Tariman, J., Karpus, W., Mehta, J. (2003). Identifying novel therapeutic targets in myeloma: CD20, CD25 and CD52 expression on malignant plasma cells [Abstract No. 5221]. Blood, 102(11), 373B-373B. Publications - Non-Peer Reviewed: 1. Tariman, J. D. (2010). Role specialization in oncology: Finding your niche. ONS Connect, 25(5), 6-9. 2. Tariman, J. D. (2010). Where to draw the line: Professional boundaries in social networking. ONS Connect, 25(2), 10-13. 3. # Tariman, J. D. (2009). Promoting testicular health: Strategies that work. Advance for Nurse Practitioners, 17(11), 18. 4. Tariman, J. D. (2009). Do you know how to care for older adults? ONS Connect, 24(12), 8-11. 5. Tariman, J. D. (2009). Mentoring in publication: A lifelong legacy. ONS Connect, 24(1), 8-12. 6. Tariman, J. D. (2009). Men and Alzheimer’s disease: Sex differences in risk. Advance for Nurse Practitioners, 17(2), 23. 7. # Lowe, J. & Tariman, J. D. (2008). Lower extremity amputation: Black men with diabetes overburdened. Advance for Nurse Practitioners, 16(11), 28. 8. # Tariman, J. D. (2008). Suicide among boys and men: More challenges ahead. Advance for Nurse Practitioners, 16(8), 25. 9. ∞Tariman, J. D. (2008). Technologic advancements in cancer care: Changing practice, changing patients' lives. ONS Connect, 23(7), 8-11. 10. # Tariman, J. D. (2008). Life span vs. healthy life expectancy: Where did men go wrong? Advance for Nurse Practitioners, 16(5), 26. 8 11. # Tariman, J. D. (2008). Obesity in men. Advance for Nurse Practitioners, 16(2), 26. 12. # Tariman, J. D. (2007). Subfertility in men. Advance for Nurse Practitioners, 15(11), 25. 13. # Tariman, J. D. (2007). Osteoporosis in men: A real threat. Advance for Nurse Practitioners, 15(8), 21. 14. # Tariman, J. D. (2007). Testosterone replacement. Advance for Nurse Practitioners, 15(2), 22. 15. ∞ Tariman, J. D. (2007). Understand substance abuse in nurses. ONS Connect, 22(8), 18. 16. ∞ Tariman, J. D. (2007). Straight talk. When should you blow the whistle for ethical reasons? ONS Connect, 22(2), 22-23. 17. ∞ Tariman, J. D. (2007). Exploring an unfamiliar world: Advice for novices delving into the research arena. ONS Connect, 22(12), 12-16. 18. ∞ Tariman, J. D. (2007). Emergency preparedness: What have we learned? ONS Connect, 22(9), 8-12. 19. # Tariman, J. D. (2007). Diabetes in minority men. Advance for Nurse Practitioners, 15(2), 18. 20. ∞ Tariman, J. D. (2006). Is your chapter on the road to success or experiencing some bumps along the way? ONS News, 21(12), 1, 4-5. 21. ∞ Tariman, J. D. (2006). ONS members bring oncology nursing-sensitive patient outcomes to the bedside. ONS News, 21(10), 1, 4-6. 22. # Tariman, J. D. (2006). Prostate cancer: Screening and early detection. Advance for Nurse Practitioners, 14(9), 20. 23. # Tariman, J. D. (2006). Smoking cessation in men. Advance for Nurse Practitioners, 14(6), 21. 24. # Tariman, J. D. (2006). Substance abuse among adolescent boys. Advance for Nurse Practitioners, 14(3), 18. 25. Berenson, J., Tariman, J. D., Bensinger, W., Doss, D., & Swift, R. A. (2004). ONS News: Spotlight on Symposia,19(9), 79-80. 26. Tariman, J. D. (2003). Performance improvement for myelosuppressed patients: Institutional case studies. In. D. Wujcik & C. Lemoine. Performance improvement in myelosuppression management: Continuing the ATAQ initiatives. Pittsburgh, PA: Oncology Nursing Society. P33-37. 27. Tariman, J. & Hammer, M. (2009). The PSONS research survey results. Puget Sound Oncology Nursing Society Quarterly, 32(2), 11, 13-14. 28. ≈ Tariman, J. D. (2008). Multiple myeloma: Maximizing the therapeutic benefits of every novel agent. Puget Sound Oncology Nursing Society Quarterly, 31(3), 1, 3-5. 29. ≈ Tariman, J. D. (2003). Clinical Update: Thalidomide in patients with multiple myeloma. Chicago Chapter Oncology Nursing Society Newsletter, Lead article, Fall 2003. 30. ≈ Tariman, J. D. (2003). IMiDs: Novel thalidomide analogue for multiple myeloma. Chicago Chapter Oncology Nursing Society Newsletter, Lead article, Summer 2003 RESEARCH Pre-Doctoral Grant 9 1. Pre-Doctoral Fellow, Ruth L. Kirschstein National Service Award funded by the National Institute of Health, National Institute of Nursing Research, Individual Fellowship Grant #1F31NR011124-01A1 Project title: Treatment decision making in older adults newly diagnosed with multiple myeloma; September 2009 – March 2011 (18 months full support). Sponsors: Barbara Cochrane, PhD, RN, FAAN (University of Washington) & Donna L. Berry, PhD, RN, FAAN (University of Washington and Dana Farber Cancer Institute). 2. Pre-Doctoral Fellow, Ruth L. Kirschstein National Service Award funded by the National Institute of Health, National Institute of Nursing Research, Institutional Grant #T32-; Training in Biobehavioral Nursing Research; September 2006 – September 2008 (full support). Mentor: Donna L. Berry, PhD, RN, FAAN Research residency: Electronic Self Report Assessment for Cancer (ESRA-C) Participated in the recruitment of participants and coded subjective data. Performed validation and testing of the Acceptability E-scale. Published manuscript related to psychometric properties of Acceptability E-scale. Graduate Thesis Doctoral dissertation: Treatment decision making in patients with newly diagnosed multiple myeloma – Fall 2008 to Winter 2011 Master’s thesis: Incidence of Nosocomial Infections in OR and ICU at Cebu Doctors Hospital: Implications for Infection Control Program. Principal Investigator - January 1992 to June 1993. Collaborative Research “QOL post-ASCT among elderly adults with multiple myeloma” Chicago Oncology Nursing Society Research Grant, October 2002 to October 2005, ($1,500 Co-Principal Investigator with Judith Paice, PhD, RN) Pharmaceutical and Cooperative Sponsored Research 10 Sub-Investigator Role: A. Assessment and monitoring of side effects and serious adverse events. B. Authorized designee to sign case report forms and documentation of response to investigational therapy (Form 1572). 1. CC-5013-MM-016 Lenalidomide Expanded Access Program for Patients with Relapsed and/or refractory MM. November 2005-March 2006 2. ECOG4A03 A Randomized Phase III Study of CC-5013 plus Dexamethasone versus CC-5013 plus Low Dose Dexamethasone in Multiple Myeloma with Thalidomide plus Dexamethasone Salvage Therapy for Non-Responders – May 2005 to March 2006 3. An Open-Label Phase II Trial of Motexafin Gadolinium (MGD) in Patients with Relapsed or Refractory Multiple Myeloma. Sub-Investigator – November 2004 to March 2006. 4. CC-5013-MM-014: A Multicenter, Single-Arm, Open-Label Study to Evaluate the Safety and Efficacy of Single-Agent CC-5013 in Subjects with relapsed and Refractory Multiple Myeloma. Sub-Investigator – July 2003 to March 2006. 5. CC-5013-MM-009: A Multicenter, Randomized, Parallel-Group, Double-Blind, Placebo-Controlled Study of CC-5013 Plus Dexamethasone in Previously Treated Subjects with Multiple Myeloma. Sub-Investigator – May 2003 to June 2004. 6. E1A00: A Randomized Phase III trial of Thalidomide Plus Dexamethasone Versus Dexamethasone in Newly Diagnosed Multiple Myeloma. Sub-Investigator – January 2003 to May 2004. 7. M34102-052: An Open-Label, Randomized Study to Assess Two different Strategies of Combining Dexamethasone with Velcade in Patients with Relapsed Multiple Myeloma who Have Failed Four or More Lines of Therapy. Sub-Investigator – January 2003 to May 2003. 8. M34101-040: An International, Non-Comparative, Open Label Study of PS-341 Administered to Patients with Multiple Myeloma Who Experienced Relapsed or Progressive Disease After Receiving At Least Four Previous Treatment Regimens or Experienced Progressive Disease After Receiving Dexamethasone in Millennium Protocol M34101-039. Sub-Investigator – February 2002 to May 2003 9. An International, Multi-Center, Randomized, Open Label Study of PS-341 Versus High-Dose Dexamethasone in Patients with Relapsed or Refractory Multiple Myeloma Protocol M34101-039. Sub-Investigator – February 2002 to May 2003 10. A phase II, Open Label, Extension Study to Provide PS-341 to Patients Who Previously Participated in a PS-341 Clinical Study and Who May Benefit from ReTreatment with or Continuation of PS-341 Therapy Protocol M34101-029. SubInvestigator – February 2002 to April 2003 11. A phase II, Open Label Study of PS-341 in Patients with Relapsed/Refractory Myeloma Protocol M34101-025. Sub-Investigator – February 2002 to June 2002 12. Randomized Phase III Study of Dexamethasone With or Without Genasense (Bcl-2 Antisense Oligonucleotide) in Patients with Relapsed or Refractory Multiple Myeloma Protocol GMY302. Sub-Investigator – February 2002 to May 2003. 11 PROFESSIONAL CONSULTATIONS 2006 – present International Myeloma Foundation North Hollywood, CA Chair – Peripheral Neuropathy Group, Nurse Leadership Board 2005 – present Men’s Health Network Washington, D.C. Member, Board of Advisors 2003 – present Millennium Pharmaceuticals Cambridge, MA Clinical Consultant Member, Speaker’s Bureau 2007 – present Kyphon, Incorporated Clinical Consultant Member, Speaker’s Bureau PROFESSIONAL PRESENTATIONS International International Society of Nurses in Cancer Care (ISNCC) Biennial Conference. Computerized Assessment for Patients with Cancer: aka Electronic Self Report Assessment-Cancer (ESRAC). Singapore City, Singapore. August 2008. XI IMW - Nursing guidelines for enhanced patient care. Poster Session, XI International Myeloma Workshop. Kos, Greece. June 2007. 9th International Myeloma Workshop-Cleveland Clinic Nursing Symposium – Management of Chemotherapeutic Side Effects, Sydney, Australia April 2005 National Conference Lectures 1. ONS Institute of Learning – Survivorship Care Issues in Patients with Myeloma. November 13, 2010. 2. ONS Congress – Changing Patient Care in Multiple Myeloma: The IMF Nurse Leadership Board’s Long-Term Survivorship Care Plan. May 13, 2010. 3. ONS Congress – Leading the Way: Managing Multiple Myeloma for the Long-term. Presenter – Novel therapies for multiple myeloma. San Antonio, TX, May 1, 2009. 12 4. ONS Congress – Navigating the clinical course of multiple myeloma patient care. Presenter – Induction therapy for patients ineligible for stem cell transplant. San Antonio, TX, April 30, 2009. 5. ONS Congress – Oncology Nursing Patterns in Multiple Myeloma. Presenter – Management of Treatment-related Effects. San Antonio, TX. April 29, 2009. 6. ONS Congress- Leading the Way: Managing Multiple Myeloma for the Long-Term. Presenter – Update on Novel Therapies. San Antonio, TX. May 1, 2009. 7. ONS Congress – Navigating the Clinical Course of Multiple Myeloma Patient Care. Presenter – Induction Therapies for Transplant Ineligible Patients. San Antonio, TX. April 30, 2009. 8. ONS Congress – Changing Paradigms in the Treatment of Multiple Myeloma: A Nurse-Centric Case-Based Discussion– Chair and Presenter, Symposium, Oncology Nursing Society Annual Congress. Philadelphia, PA. May 17, 2008 9. ONS Congress – Staying Upright: Managing Spinal Complications for Patients with Cancer – Symposium, Oncology Nursing Society Annual Congress. Philadelphia, PA. May 17, 2008 10. ONS Congress – The IMF Nurse Leadership Board Consensus of Care: New Insights on Novel Therapies in Multiple Myeloma – Symposium, Oncology Nursing Society Annual Congress. Philadelphia, PA. May 16, 2008 11. ONS Congress – The Spectrum of Multiple Myeloma Patient Care: A Case-Based Assessment for Oncology Nurses – Symposium, Oncology Nursing Society Annual Congress. Philadelphia, PA. May 18, 2008 12. ONS Congress - Management of Novel Therapeutics’ Side Effects – A Nurse Centric Model. Symposium, Oncology Nursing Society Annual Congress. Las Vegas, NV. April 2007 13. WIN Conference – Decision Making in Oncology Settings and Validation and Testing of the Acceptability E-scale. Poster Session, Western Institute of Nursing Annual Conference. Garden Grove, CA. April 2008. 14. ONS Congress – Updates on metastatic spine disorders. Symposium. ONS Annual Congress. Las Vegas, NV. April 2007. 15. NCNRC - Quality of life (QOL) in older adults undergoing autologous stem cell transplant (ASCT). Poster Session, National Cancer Nursing Research Conference. Hollywood, CA. February 2007 16. ONS Annual Congress – Frontline Therapies for Multiple Myeloma, Orlando, FL April 2005 17. ONS Annual Congress - Multiple Myeloma: A New Treatment Paradigm, Anaheim, CA April 2004 18. ONS Fall Institute – Proteasome Inhibition in Multiple Myeloma, Philadelphia, PA November 2003 19. ONS Annual Congress - Bortezomib for Relapsed and Refractory Multiple Myeloma – Denver, CO April 2003 Regional Conference Lectures 13 1. Annual Scripps Advanced Practice Nurses Oncology Conference. Presenter – Plasma cell Disorders. San Diego, CA. February, 2009. 2. Annual NW Oncology Conference - Advances in Multiple Myeloma. Annual “Concepts in Oncology Conference". Symposium, Boise, ID. October 2007 3. Annual Scripps Cancer Center Oncology Conference – Novel Therapies for Patients with Multiple Myeloma and Nursing Management of Common Toxicities, San Diego, CA September 2007 4. Myeloma: Disease overview, treatment considerations and nursing implications. Orlando, FL. January 2006 5. Myeloma: Disease overview, treatment and clinical update on bortezomib therapy. Milwaukee, WI, December 2005 6. International Myeloma Foundation – Patient and Family Seminar – QOL Session, Northbrook, IL June 2005 7. Scripps’s Cancer Center Annual Advanced Practice Nurse Conference – Advances in Multiple Myeloma, San Diego, CA March 2005 8. Millennium Pharmaceutical Nurse/Pharmacist Consultant’s Meeting – Clinical Update on Bortezomib in Hematologic Malignancies, Beverly Hills, CA November 2004 9. Cornell-NYU/Letters& Science Annual Oncology Conference – Advances in Hematologic Malignancies, New York City, NY October 2004 10. Northwestern Memorial Hospital’s Annual Oncology Nursing Conference – Program Chair and Presenter: Novel Agents for Multiple Myeloma, Chicago, IL October 2004 11. Annual Oncology Care Conference-“Novel Therapeutic Options for Myeloma”, Honolulu, HI July 2004 12. Chicago Myeloma Foundation - Symptom Management in Patients with Multiple Myeloma, Chicago, IL September 2003 Online Educational Programs 1. Christiansen, N., Bensinger, W., Colson, K., Harvey, R. D., Lonial, S., & Tariman, J. D. (2009). Module 8: Building the best myeloma regimens: Is there a blueprint? Available at http://www.questmeded.com/cebin/owa/pkg_disclaimer_html.display?ip_mode=secure&ip_company_code=P4CC&i p_test_id=14504&ip_cookie=32248899 2. Tariman, J. D. & Harvey, R. D. (2009). Module 2: Clinical Considerations and Implications for Optimizing Patient Outcomes in Multiple Myeloma. Available at http://www.clinicaloptions.com/Oncology/Management%20Series/Multiple%20Myel oma.aspx 3. Harvey, R. D & Tariman, J. D. (2009). Module 1: Mechanisms of Action and Clinical Advances in the Treatment of Multiple Myeloma. Available at http://www.clinicaloptions.com/Oncology/Management%20Series/Multiple%20Myel oma.aspx 4. Tariman, J. D., Harvey, R. D. & Colson, K. (2009). Module 6: Multiple Myeloma: Relapsed/Refractory Disease – Care Team Considerations. Available at http://www.questmeded.com/ce- 14 5. 6. 7. 8. bin/owa/pkg_disclaimer_html.display?ip_cookie=31242293&ip_mode=secure&ip_te st_id=14409&ip_company_code=P4CC Tariman, J. D. & Harvey, R. D. (2009). Module 4: Multiple Myeloma Induction and Transplant Strategies – Care Team Considerations. Available at http://www.questmeded.com/cebin/owa/pkg_disclaimer_html.display?ip_cookie=31242245&ip_mode=secure&ip_te st_id=13569&ip_company_code=P4CC Tariman, J. D., Fonseca, R., & Colson, K. (2008). Changing Paradigms in the treatment of Multiple Myeloma: Nurse-Centric Case-Based Discussions. Available at http://old.imeronline.com/315_mm/home.html Lonial, S. & Tariman J. D. (2008). Clinical Updates in the Management of Patients With Multiple Myeloma Case-Based DVD. Available at http://www.thecbce.com/onlineEdu_detail.aspx?act_id=258 Smith, L., Tariman, J. D., & Harvey, R. D. (2008). Module 2: Diagnosis and Staging of Multiple Myeloma (MM) and Front-Line Therapy for the Transplant-Ineligible Patient: Care Team Considerations. Available at http://www.questmeded.com/cebin/owa/pkg_disclaimer_html.display?ip_cookie=31242277&ip_mode=secure&ip_te st_id=13567&ip_company_code=P4CC Institutional Lectures 1. Harborview Medical Center – Multiple Myeloma: Maximizing the Therapeutic Benefits of Every Novel Agent. Seattle, WA. December 09, 2008. 2. FHCRC – Symptom Management and Nurse’s Role in Patient Care. International Myeloma Foundation Patient and Family Seminar. Seattle, WA. January 2008. 3. FHCRC- Management of Renal Insufficiency in Patients with Multiple Myeloma. Fred Hutchinson Cancer Research Center Nursing Education Day. Seattle, WA. January 2007. 4. NW Oncology Cancer Center - Vertebroplasty and kyphoplasty in patients with metastatic bone disease. Olympia, WA. February 7, 2007. 5. Fort Collins-ONS Chapter – “Multiple Myeloma, Disease Overview and Advances in Therapy”, Fort Collins, CO. June 28, 2006. 6. Lutheran General Hospital- “Nursing Management of Patients with Multiple Myeloma”, Park Ridge, IL. March 3, 2006. 7. Kaiser-Permanente Oncology – “Nursing Management of Patients with Multiple Myeloma”, Portland, OR. January 19, 2006. 8. Palm Beach Myeloma Support Group – “Advances in Myeloma Therapy”, Palm Beach, FL May 6, 2005 9. Mid Chesapeake Bay ONS- “Novel therapies for Patients with Myeloma”, Washington, D.C. November, 2004 10. UPMC Cancer Center- “Proteasome Inhibition-A Novel Option for the Treatment of Myeloma”, Pittsburgh, PA, June 10, 2004 11. Chicago ONS Chapter - Multiple Myeloma Update, Chicago, IL March 2004 12. Waukesha Memorial Hospital - Multiple Myeloma: Overview and Novel Therapeutic Agents, Waukesha, WI May 2003 15 HONORS AND AWARDS 2008 Best Student Poster Award Western Institute of Nursing Conference, Garden Grove, CA 2008 2007 Best Poster Award National Cancer Nursing Research Conference, Hollywood, CA 2007 2006 Achievement Rewards for College Scientists Seattle, WA 2006 Betty Irene and Gordon Moore Fellowship, UCSF San Francisco, CA 2005 Outstanding New Member Award Oncology Nursing Society (ONS) Chicago Chapter Chicago, IL 1991 Academic Excellence Award 13th Place, Philippines Board of Nursing Examination Manila, Philippines 1991 Cum Laude BSN Class 1991 Cebu, Philippines PROFESSIONAL SERVICES 2008 – present Editorial board member – The Oncology Nurse 2006 – 2010 Contributing writer – Advance for Nurse Practitioners-Men’s Health Column 16 2005 – 2010 Contributing editor - ONS News 2005 – 2009 Continuing Education Pilot Reviewer – ONS ECCIT 2003 – present Review board member - Clinical Journal of Oncology Nursing 2004 – present Review board member - Oncology Nursing Forum 2004- 2006 Chicago Oncology Nursing Society – Board of Director and Research Committee Member 2002 – 2006 Reviewer - Northwestern University Clinical Protocol and Scientific Review Monitoring Committee PROFESSIONAL ORGANIZATIONS Council for the Advancement of Nursing Science Member 2009-present Sigma Theta Tau Honors Society Member 2008 – present Western Institute of Nursing Member 2007-present Oncology Nursing Society Member and Contributing Editor for 1998- present ONS Connect (2005-2010) 17 Puget Sound Chapter Oncology Nursing Society Chair, Research Committee 2006 - 2010 Chicago Chapter Oncology Nursing Society Director-at-large 2001-2006 PROFESSIONAL TEACHING: 1992-1995 Specialization: Adult Medical-Surgical Nursing – Three years of classroom and clinical instruction, BSN program Cebu Doctors’ University Philippines Invitational Speech March 2006 Commencement Speaker University of the Visayas College of Nursing Cebu, Philippines Community or Public Services Activities: 1. MM Fighters Seattle Patient Support Group, Guest speaker. Topic: Treatment Decision Making and Quality of Life. January 2008. 2. Chicago Gilda’s Club – Support Group Facilitator, January 2006. 3. Men’s Health Network – Board of Directors, Member – July 2005 to present 4. Multiple Myeloma Research Foundation – Volunteer, fundraising activities. Provides free lectures to patients and family members – March 2002 to March 2006. 5. International Myeloma Foundation – Volunteer, fundraising activities. Provides free lectures to patients and family members March 2002 –March 2006. 6. Medical Mission to Haiti – Health care volunteer, provided basic health care services to the underserved children and families in Port-au-Prince, Haiti – July 2000.