Advanced Distillation Lab
advertisement

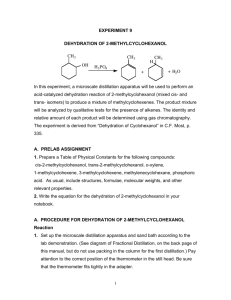
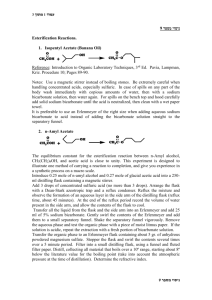
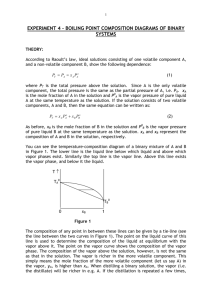
Problem: How does a difference in physical properties make it possible to separate the components of a solution, a homogeneous mixture? How can we use this to isolate and identify various substances? Introduction: Chapter one of our textbook discusses matter and the various physical and chemical changes it can undergo. The chapter also points out that pure substances can be separated from each other on the basis of the differences in their physical and chemical properties. Distillation is an example of a physical process that can be used to separate two miscible liquids from each other. The separation occurs because each of the liquids boils at a different temperature. Liquids with low boiling points are said to be more ”volatile” while those with high boiling points are called less “volatile”. In a simple distillation, the mixture is heated until the solution boils, giving off the vapor of the component with the lower boiling point (the more volatile of the two). The vapor is directed through a tube cooled by water (known as the condenser) into another container (called the receiver). Gentle heating is continued until all, or most, of the more volatile liquid has evaporated and been condensed in the receiver. The pure liquid collected in this fashion is called the distillate. If there are only two liquids in the mixture, distillation is complete at this point. In more complex situations, such as the separation of crude oil into the various petroleum fractions, a more elaborate separation and collection process is necessary. Since distillation involves no change in the identity of either component, it is a physical process, rather than a chemical one. You will carry out two fractional distillations, then do some tests of the physical properties of the distillate to help you confirm the separation of the homogeneous mixture and the identity of the substance. Materials: 1. Side-arm distilling flask 2. Bunsen Burner 3. Watch Glass 4. Distillation Mixture 5. Boiling stones chips 6. Test Tube 7. Thermometer 8. Graduated Cylinder 9. Glass bend and tubing 10. Stoppers 11. Balance 12. 125 mL Erlenmeyer flask 13. 400 mL Beaker Safety: Do not allow open flame to directly touch distilling apparatus Use great care when handling the distilling flask and condenser Do not allow all liquid to evaporate form the distillation flask Procedure: 1. Place a boiling chip in the 125mL distilling flask and add 40 mL of the unknown mixture to the flask. Note the odor and appearance of the mixture and record your observations in a Data Table. 2. Attach the flask condensing arm to the test tube via a glass bend and vinyl tubing. Insert the stopper with the thermometer into the top of the flask. Be sure that the tip of the thermometer extends below the side-arm of the flask. This ensures the best possible contact of the distilling vapors. 3. The flask should be submerged in the water present in the 400 mL beaker. Place the receiving test tube tube in an ice bath within the Erlenmeyer flask. 4. Begin heating slowly, taking temperatures every 30 seconds. 5. Observe carefully what happens as the liquid begins to boil. In your Data Table, note the temperature reading when the first bubbles start to rise from the boiling stones. Observe the distillation process, noting in particular the way the vapors can be seen to recondense in the neck of the flask. 6. After you have collected about 10 mL of liquid, remove the test tube and label it #1. Place a new test tube to catch the distillate. Continue to collect 10 mL samples of the distillate, placing them in a beaker and labeling each beaker. When the substance undergoes the next temp increase collect 5 mL of the new liquid. 7. Restart the process for the next mixture. 8. Place 4-5 drops of each distillate on a watch glass and test for flammability. Compare to the original mixture. Analysis: 1. Prepare a data table for collection of temperatures and observations. 2. Graph the temperatures vs. time of each mixture 3. Provide a written description of the shape of your graph. 4. How did the flammability of each of the distillates compare to each other and to the original solution? 5. During distillation, the temperature of the escaping liquid remains relatively constant while most of the liquid distill. This temperature is the boiling point of the distillate. a. At what temperature did most of the unknown distill in each experiment? b. What is the boiling point of the second substance in the solution? c. How does this compare with the boiling point of pure water, 100˚C? d. What can you conclude about the identity of the liquid left in the flask? e. Perform a density test for all distillates. Use this data, combined with your boiling point data to identify the substances. Conclusion: Explain the concept of separation of mixtures by physical processes, such as distillation. Compare how this process did not separate the pure substances that made up the homogeneous mixture. Discuss the results of your lab and how you could improve on your lab technique