Neuroscience Gene Vector and Virus Core
advertisement

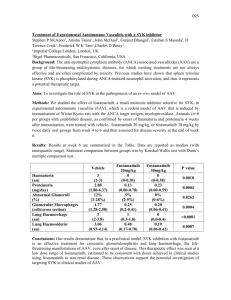
Neuroscience Gene Vector and Virus Core Virus request form for AAV. Fill out form and e-mail to mlochrie@stanford.edu Date: . Investigator: . Lab head: . AAV capsid desired (e.g., AAV-1, AAV-2, etc.): . Production scale desired (i.e., number and size of flasks of cells to be transfected to produce the virus? Transfection of two to five T-225s is often used to produce AAV. However scales ranging from one well of a 96 well plate to 100 T-225s are possible. Transfection of one to two T-225s are the minimum recommended if the virus is to be used in vivo so that the final virus concentration is high.): _________________ . (Alternatively you can request a specific volume and concentration.) Purity level desired (crude or iodixanol gradient-purified?): . (Crude AAV can be used for many cell culture experiments and is less expensive than purified but since it is a whole cell lysate it can be toxic to cells and may need to be titrated to determine if the desired biological effect can be obtained before toxic effects occur. For in vivo use, only gradient purified AAV should be used.) Name of transgene vector (e.g., pAAV CMV-GFP): . Description of transgene vector (e.g., AAV-2 ITR vector with GFP gene driven by CMV promoter): . Does your vector encodes oncogenes, select agents, toxins, apoptotic genes, etc. that may be more hazardous than usual? (yes/no) . If your vector encodes a fluorescent reporter, will the promoter driving reporter gene expression be active in 293 cells? (yes/no)? . (such that transfection and infection efficiencies can be assessed) Are you preparing the vector plasmid (yes/no)? . If yes, at least 30 g plasmid is needed per T-225 transfected. The DNA should be high quality (e.g., purified using ion exchange, not silica-based columns). The exact plasmid prep to be used for transfection to produce virus should be well characterized (e.g., sequenced, digested with several restriction enzymes, functional tests if possible). Do you have a complete predicted sequence for your vector (yes/no)? . If yes, please e-mail a text or Vector NTI (preferred) file with as much information about landmarks as is available. The sequence is used to determine a) if the viral genome encoded by the plasmid is oversized and b) if the viral genome can be detected by Q-PCR probes that are in-stock (pCMV, hGH poly A, WPRE) for genomic titering. [Oversized genomes do not produce virus or transduce cells as well as those that are undersized.] Does your vector encode a single-stranded or double-stranded (i.e., self-complimentary) genome? _____________________. This is important because self-complimentary genomes are difficult to titer by Q-PCR and have half the packaging capacity of a single-stranded genome. What characterization of the final virus preparation do you desire: infectious titer by infection of 293 cells (yes/no)? . (applicable only if genome encodes a fluorescent reporter that is expressed in 293 cells) genome titer by Q-PCR detection of viral genome (yes/no)? . (The genome titer of AAV is what is traditionally determined and reported in publications even though an infectious titer is more relevant.). Probe that can be used for Q-PCR (pCMV, hGH poly A, or WPRE): . (If the AAV genome does not contain one of these elements then a probe that will detect the genome may need to be ordered which will result in additional cost.) Would you like the final virus preparation aliquoted and if so what size? ________ . (Although AAV is very stable it is best to aliquot to minimize repeated freezing and thawing. Final volumes may be from 20-100+ microliters depending on the scale of production. Usually 2-10 microliter aliquots are made. ) What date do you need the virus by? Notes or comments: . .