File
advertisement

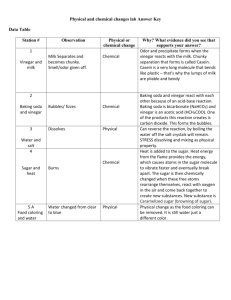
Theoretical Background What is vinegar and what does it contain? Vinegar is a sour-tasting, clear liquid that freezes below 62 °F. It is known as White Vinegar, Ethanoic Acid, or Acetic Acid. It is an organic chemical compound that consists mostly of acetic acid (CH3COOH) and water (H2O). It is made naturally and synthetically, through the oxidation of Ethanol in an alcoholic liquid. The methods that can be used to analyze the content of vinegar. The easiest method to determine the acid content of vinegar would be a process known as the Acid Base Titration Analysis. Here, an identified volume of HCl would be measured with a graduated cylinder and titrated with a solution of NaOH of known molarity. Afterward, the solution would be added slowly until all the acid is neutralised. To accurately measure the volume of NaOH used, the exact amount of NaOH required to neutralised must be known. This can be done using an acid-base indicator, otherwise known as Phenolphthalein, which is colourless in acidic solutions but turns pinkish in a basic solution. By following these procedures, the acid content of the vinegar can be found. Titration- what is it? How does it work? Titration is a laboratory technique used to determine the concentration of an unknown reagent using a standard concentration of another reagent that reacts chemically with the unknown reagent. The standard solution would then be referred to as the ‘titrant’. It works by adding a specific reactant to the solution until it is neutralised. The whole point is to determine the amount of reactant required to neutralise or bring the solution to a known pH. Indicator- what is it? How does it work? An indicator is a weak (usually organic) acid that changes its molecular shape with changes in pH. As the shape changes, so does the colour of the solution. Phenolphthalein has a figure below pH8, and is virtually colourless. At a pH above 8, however, the shape changes and turns to a shade of pink. Therefore it is colourless in acid and pink in a base, which can be used to determine the acidity of solution. Equivalence point and end point – what are they? The equivalence point is the point where the number of moles of base equal the number of moles of acid. The end point is the point where the used indicator changes colour, otherwise known as the ‘indication point’. Hypothesis: My hypothesis is that based on this research, the higher the concentration of the vinegar inside the solution, the higher the concentration of Ethanoic Acid will be. This is given that the concentration of the Sodium Hydroxide is kept fixed. Data Collection Organized Raw Data Vinegar (ml) H2O (ml) 0.5 1 1.5 2 2.5 50 50 50 50 50 NaOH (ml) Trial 1 Trial 2 Trial 3 8.1 8.2 10 26.7 24 19 27.5 27.5 27.2 35.7 31 34.7 50.5 47.8 44.4 Table 1: Title Data Processing Write the Chemical Equation CH3COOH + NaOH CH3COONa + H2O Calculation from Chemical Equation Table 2: Title NaOH Vol (dm3) 0.0081 0.0082 0.01 M (NaOH) Mol (NaOH) 1 0.0081 1 0.0082 1 0.01 0.0267 0.024 0.019 1 1 1 0.0267 0.024 0.019 0.0275 0.0275 0.0275 1 1 1 0.0275 0.0275 0.0275 0.0357 0.031 0.0347 1 1 1 0.0357 0.031 0.0347 0.0505 0.0478 0.0444 1 1 1 0.0505 0.0478 0.0444 Vinegar Vol (dm3) 0.5 0.5 0.5 Average 1 1 1 Average 1.5 1.5 1.5 Average 2 2 2 Average 2.5 2.5 2.5 Average Mol (Vinegar) 0.0081 0.0082 0.01 Vinegar Solution Vol (dm3) 0.0505 0.0505 0.0505 0.0267 0.024 0.019 0.051 0.051 0.051 0.0275 0.0275 0.0275 0.0515 0.0515 0.0515 0.0357 0.031 0.0347 0.052 0.052 0.052 0.0505 0.0478 0.0444 0.0525 0.0525 0.0525 M (Vinegar) 0.16 0.16 0.20 0.17 0.52 0.47 0.37 0.46 0.53 0.53 0.53 0.53 0.69 0.60 0.67 0.65 0.96 0.91 0.85 0.91 Data Presentation Graph 1: Concentration Chart 1.2 1 0.8 Trial 1 0.6 Trial 2 Trial 3 0.4 0.2 0 0.5 1 1.5 2 2.5 Graph 2: Concentration Graph 0.8 0.7 0.6 0.5 Trial 1 0.4 Trial 2 Trial 3 0.3 0.2 0.1 0 0.5 1 1.5 2 Explanation Analyzing Results Based on the results attained, it proves that the more the Vinegar in a solution, the higher the concentration of the Ethanoic acid will be. This completely supports the original hypothesis. The experiment was also done in 3 trials for each amount of vinegar, which means that results are generalised. Based on the concentration graph, Trial 3 has the best and clearest result. There no anomalies, as all the results make perfect sense. Results show that as the amount of the vinegar inside the solution increase, as does the concentration of the Ethanoic Acid. The experiment was done with a fixed amount of Phenolphthalein, Water, and a fixed concentration of Vinegar and Sodium Hydroxide. There were no anomalies, because as seen in the table, chart, and graph, the concentration of the Ethanoic Acid never decreases if the volume of the Vinegar inside the solution is increased. This is because the amount of water is kept at a fixed volume, but the volume of the vinegar varies. Evaluation Method Evaluation The method of the experiment was very good. It was executed properly as well. For future reference however, this experiment was done in a rush. Though done carefully as to attain proper results, it would be better to take things at a considerably slower pace next time, to even better ensure accuracy. Data Reliability Data presented is very reliable. All note takings were done cautiously and carefully so that the data collected would be accurate. All calculations were double checked to make sure it is accurate. The formula was taken directly from notes taken in class. Balancing the formula was also done carefully. Everything, no matter at what pace, was done with careful work, so results are most likely to be accurate and reliable. No anomalies present. Conclusion The conclusion here is that based on the data collected, processed, and presented, it’s fair to say that the higher the amount of Vinegar in a solution, the higher the concentration of the Ethanoic Acid. It is most clearly shown with the graph, trial 3. It has a steady inclination, proving the steady increase in concentration of Ethanoic Acid. During the experiment, 2 batches of NaOH were used. That was the only problem and uncertainty faced during the procedures. Hopefully though, the concentration of NaOH was exactly the same for both batches. Otherwise anomalies might have shown and would be identified. Other than that, the method seems practical, logical, reasonable, and reliable. Perhaps next time a good research question would be to find out what would happen should the concentration of NaOH be altered during the experiment. Still, from this experiment, the concluding statement would be that the concentration of Vinegar is directly proportional to the concentration of Ethanoic Acid. This supports the hypothesis, and evidence supporting conclusion is presented in the table, chart, and graph. References "ACID_BASE INDICATORS." ACID_BASE INDICATORS. N.p., n.d. Web. 02 Feb. 2013. "Acid-base Indicators." Acid-base Indicators. N.p., n.d. Web. 02 Feb. 2013. "The Analysis of Vinegar." The Analysis of Vinegar. N.p., n.d. Web. 02 Feb. 2013. "ChemLab - Techniques - Titration." ChemLab - Techniques - Titration. N.p., n.d. Web. 03 Feb. 2013. Hutabarat, Kusumawati. METHOD - Stoichiometry. N.p.: n.p., n.d. Print. "Titration Analysis of Vinegar." Titration Analysis of Vinegar. N.p., n.d. Web. 03 Feb. 2013. "Vinegar FAQs: From Balsamic to Apple Cider to Red Wine to Distilled." Vinegar FAQs: From Balsamic to Apple Cider to Red Wine to Distilled. N.p., n.d. Web. 03 Feb. 2013. "What Is the Chemical Composition Of A Vinegar?" About.com Chemistry. N.p., n.d. Web. 02 Feb. 2013. "What Kind of Acid Does Vinegar Have?" Yahoo! Answers. Yahoo!, n.d. Web. 02 Feb. 2013.