Scientific Measurement Notes Chemistry I Accuracy, Precision, and
advertisement

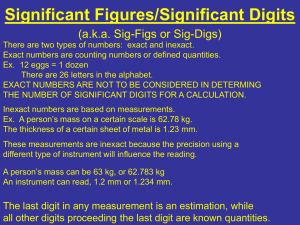
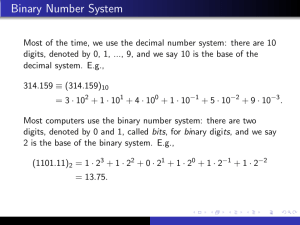
Scientific Measurement Notes Chemistry I Accuracy, Precision, and Error (pg 64-65) 1) Define accuracy in your own words. Getting the accepted answer. 2) Define Precision in your own words. Getting similar answers on repeated attempts. 3) Describe a situation when accuracy would be more important than precision. When calculating an amount is not important, but a yes or no is important—pregnancy test When you only have one shot, so repeatability is not relevant—last minute shot at a basketball game or Hail Mary pass in a football game Hunting—want to fatally wound animal, but where individual shots land is not relevant Darts—want to hit the bulls eye at least once. Where other darts land is not important. 4) Describe a situation when precision would be more important than accuracy. When the relative values are important—the watches of bank robbers. As long as they’re all synced, in doesn’t matter if the time matches the time on anyone else’s clock or watch. Cutting down a tree with an ax. The exact position of the cuts is not very important. That they’re all close together is. Grades—want to be consistent 5) What might cause a set of measurements to be inaccurate? The equipment is off, for example a scale is not zeroed before it’s used. Chemicals are mixed improperly. If you’re adding the same amount of Chemical A to 100 test tubes, but Chemical A is not the proper strength, the results of the experiment may still be precise, but not the expected value. Some force is influencing all the measurements. A calculation of the stopping distance of a car done without considering friction may have high repeatability when tested, but friction will change the final stopping distance for each trial. 6) What might cause a set of measurement to be imprecise? Using different quantities of a chemical in a reaction when you should have used the same quantity for all. For example, if you are supposed to add 5 mL of Chemical A to each test tube, but you estimated instead of carefully measuring, some test tube may only get 3.6 mL and some got 7.0 mL. The final results may change with the change in amount of Chemical A. Not measuring carefully. 15 Mystery Tubes were made from three 5 ft pieces of PVC. No matter if I had measured carefully or measured sloppily, the average length of the tubes would be the same, so the accuracy would be the same no matter how well I measured. How carefully I measured would impact only the precision. 7) The formula for Percent Error is: [Absolute value (observed – accepted) / accepted] x 100 8) What is the consequence of the numerator being an absolute value to the outcome? (What would change if the formula was simply the difference of the observed and expected values?) If you were to consider if the observed value was greater than or less than the accepted value, you could then have percent error values that bear positive or negative signs. Significant Figures in Measurements (pg 66-67) 9) Make a list of the rules for determining the number of significant figures in your own words. 1. 2. 3. 4. 5. 6. If it’s not a 0, it’s significant 0s between non-0s are significant If only 0s are to the left of a non-0, those 0s to the left are NOT significant 0s tacked on the end (right) after a decimal are always significant 0s on the end (right) WITHOUT a decimal are not significant When counting (e.g. 76 sheep; 14 cars) or with an established unit (e.g. 60 seconds in a minute) there are an unlimited number of significant figures. These exact figures do not play into the rules of rounding. 10) Look up the men’s and women’s gold medal winning times for the 100 meter run in the 1948 Olympics. Then look up the men’s and women’s gold medal winning times for the same race in the 2000 Olympics. Why are more digits to the right of the decimal recorded for the 2000 Games? Why might this be important? In 1948, times were measured to tenths of seconds. In 2000, times were measured to hundredths of seconds. This allows measurements to be more accurate, and as runners become faster and faster, and differences between placings become smaller, clear placings can be established. This is the result of better technology. This change has to do with accuracy, not precision. The runners are not trying to all finish at a certain time… Significant Figures in Calculations (pg 68-71) 11) What are the rules for rounding in regards to significant figures, in your own words? Decide the number of significant figures it should have Round using the digit just beyond that point as a guide 12) What does it mean that a calculated answer cannot be more precise than the least precise measurement from which it was calculated? When might this make a difference in the real world? If I measure the area of a shape using a tool that can measure to the 1/10 of a meter, but when I complete the calculation of area, I get an answer with three places after the decimal, I should have a red flag in my mind. It doesn’t make sense that, using a tool that can measure to 1/10 of a meter at best, I can have an answer to the 1/1000 of a meter. 13) In your own words, write the rule for rounding when adding and subtracting to get appropriate significant figures. Look for the measurement with the least number of decimal places. Round your final answer to this number decimal places. 14) Again, in your own words, write a rule for rounding when multiplying and dividing to get appropriate significant figures. Look for the measurement with the least number of significant figures, regardless of decimal places. Round you answer to this number of significant figures