Application for an Amendment or Renewal to a Project Authorisation
advertisement
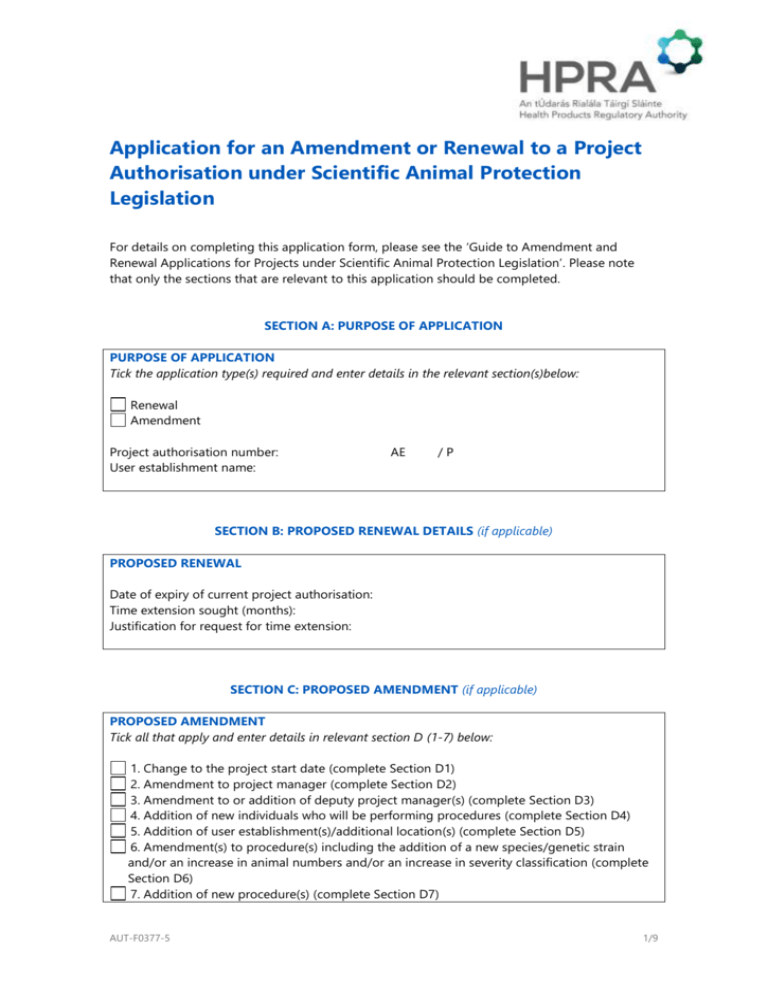
Application for an Amendment or Renewal to a Project Authorisation under Scientific Animal Protection Legislation For details on completing this application form, please see the ‘Guide to Amendment and Renewal Applications for Projects under Scientific Animal Protection Legislation’. Please note that only the sections that are relevant to this application should be completed. SECTION A: PURPOSE OF APPLICATION PURPOSE OF APPLICATION Tick the application type(s) required and enter details in the relevant section(s)below: Renewal Amendment Project authorisation number: User establishment name: AE /P SECTION B: PROPOSED RENEWAL DETAILS (if applicable) PROPOSED RENEWAL Date of expiry of current project authorisation: Time extension sought (months): Justification for request for time extension: SECTION C: PROPOSED AMENDMENT (if applicable) PROPOSED AMENDMENT Tick all that apply and enter details in relevant section D (1-7) below: 1. Change to the project start date (complete Section D1) 2. Amendment to project manager (complete Section D2) 3. Amendment to or addition of deputy project manager(s) (complete Section D3) 4. Addition of new individuals who will be performing procedures (complete Section D4) 5. Addition of user establishment(s)/additional location(s) (complete Section D5) 6. Amendment(s) to procedure(s) including the addition of a new species/genetic strain and/or an increase in animal numbers and/or an increase in severity classification (complete Section D6) 7. Addition of new procedure(s) (complete Section D7) AUT-F0377-5 1/9 SECTION D: PROPOSED AMENDMENT DETAILS (if applicable) D1. CHANGE TO THE PROJECT START DATE This section should only be completed if procedures on animals have not commenced within one year of the issue date of project authorisation. Issue date of project authorisation: Proposed date of commencement of animal procedures: Provide reason(s) as to why procedures on animals did not commence within one year of the issue date of project authorisation: Provide information on how it was ensured that no alternatives to the use of live animals have been made available since the project was first authorised. Provide information on how it was ensured that this work has not been carried out elsewhere since the project was first authorised. If this is a duplication of a previous study, justify why this duplication is necessary. D2: AMENDMENT TO PROJECT MANAGER Enter the details of the proposed project manager below: Title: First name: Address 1: Surname: Address 2: Address 3: County: E-mail: Individual authorisation number: AE Telephone: /I If no current individual authorisation number exists, please state the date of application for an individual authorisation: Please append CV (setting out education, training, experience and publication history). (A template CV is available on the HPRA website if you wish to use it.) D3: AMENDMENT TO OR ADDITION OF DEPUTY PROJECT MANAGER(S) This section can be expanded by copying and pasting as many times as required. For multiple amendments/additions, select the entire table and copy and paste as required. Title: AUT-F0377-5 2/9 First name: Address 1: Surname: Address 2: Address 3: County: E-mail: Individual authorisation number: AE Telephone: /I If no current individual authorisation number exists, please state the date of application for an individual authorisation: State the reason for amendment to deputy project manager e.g. addition of an additional deputy project manager/replacement of deputy project manager stating full name and individual authorisation number of replaced person? Please append CV, setting out education, training, experience and publication history. (A template CV is available on the HPRA website if you wish to use it.) D4. ADDITION OF NEW INDIVIDUALS WHO WILL BE PERFORMING PROCEDURES For multiple amendments, select the entire table and copy and paste as required. FIRST NAME SURNAME INDIVIDUAL If no current individual authorisation AUTHORISATION number is held, state the date of NUMBER application for an individual authorisation D5. ADDITION OF USER ESTABLISHMENT(S)/ADDITIONAL LOCATION(S) In the case of a collaboration, list the user establishment authorisation number of the new user establishment(s) at which project work is planned to take place: USER ESTABLISHMENT NAME USER ESTABLISHMENT AUTHORISATION NO. If an additional user establishment is being added please outline the reasons for the addition: If an additional user establishment is being added, ensure the compliance officer for the additional user establishment signs the appropriate section of the declaration in Section F. In the case of an amendment to a location other than the authorised user establishment(s) where procedures are planned to be carried out, list the additional location(s) where procedures AUT-F0377-5 3/9 are planned to be carried out and provide a scientific justification as to why each additional location is necessary: D6. AMENDMENT(S) TO PROCEDURE(S) INCLUDING THE ADDITION OF A NEW SPECIES/GENETIC STRAIN AND/OR AN INCREASE IN ANIMAL NUMBERS AND/OR AN INCREASE IN SEVERITY CLASSIFICATION For multiple amendments, select the entire table and copy and paste as required. Approved procedure (as listed in the project authorisation) being amended Nature of amendment including details on any increase in animal numbers for this procedure Justification for amendment Provide details on any impact to adverse effects, severity or humane endpoints this amendment may have. 1. 2. 3. Adverse effects Severity Humane endpoints Please append the currently approved project protocol with the proposed amendments highlighted in yellow or added as tracked change. The specific details for each procedure must be provided in the project protocol. Has this amendment(s) been approved by an ethics committee? Yes No If ‘yes’ please provide a copy of the ethical review application and associated approval documentation from the relevant ethics committee as outlined in the guide to projects. If ‘no’ please provide justification as to why an ethical review was not performed? If the amendment includes the addition of a new animal species/genetic strain, please answer the questions and complete the tables below. Have the animals to be used in this project been bred for specific use in scientific procedures: Yes No If ‘no’ please provide scientific justification for the reasons the animals were not specifically bred for use in procedures? Have the animals to be used in this project been taken from the wild? Yes No If ‘yes’ please provide scientific justification for the reasons a wild animal is required? AUT-F0377-5 4/9 Are the animals to be used in this project stray or feral animals of a domestic species? Yes No If ‘yes’ please provide scientific justification for the reasons a stray or feral animal of a domestic species is required? Are the animals to be used in this project an endangered species? Yes No If ‘yes’ please provide scientific justification for the reasons an endangered species is required? N.B. - if total animal numbers have increased as a result of this amendment(s) please ensure to complete Section E of this form. Species Life stage Strain/breed Genetic status Genetic alteration (if GA) Breeder/supplier establishment authorisation number Country of origin* Have any individual animal(s) proposed for use in this project been previously used in a scientific study? If yes, describe the cumulative effect of the procedures on the animal(s)? Number of animals to be used Yes No * If animal(s) are sourced outside of the Republic of Ireland, please indicate the source of the animals and provide a certificate confirming authorisation and registration of the supplier establishment (as required under Directive 2010/63/EU) where animal(s) were bred / supplied in the country of origin. D7. ADDITION OF NEW PROCEDURES For multiple additions, select the entire table and copy and paste as required. Procedure number Species Strain/breed Life stage Technique Duration of procedure Frequency of procedure Pain-relieving methods and/or anaesthesia Proposed severity classification AUT-F0377-5 5/9 Humane endpoints Number of animals to be used Adverse effect(s) of procedure on animal welfare What is the fate of the animals at the end of the procedure? If the fate of the animals is euthanasia, please state the method of euthanasia Provide detailed justification as to why it is necessary to add this new procedure: Please append the currently approved project protocol with the proposed amendments highlighted in yellow or added as tracked change. The specific details for each procedure must be provided in the project protocol. Has this amendment(s) been approved by an ethics committee? Yes No If ‘yes’ please provide a copy of the ethical review application and associated approval documentation from the relevant ethics committee as outlined in the guide to projects. If ‘no’ please provide justification as to why an ethical review was not performed? If the amendment includes the addition of a new animal species / genetic strain, please answer the questions and complete the tables below. Have the animals to be used in this project been bred for specific use in scientific procedures: Yes No If ‘no’ please provide scientific justification for the reasons the animals were not specifically bred for use in procedures? Have the animals to be used in this project been taken from the wild? Yes No If ‘yes’ please provide scientific justification for the reasons a wild animal is required? Are the animals to be used in this project stray or feral animals of a domestic species? Yes No If ‘yes’ please provide scientific justification for the reasons a stray or feral animal of a domestic species is required? Are the animals to be used in this project an endangered species? Yes No If ‘yes’ please provide scientific justification for the reasons an endangered species is required? Note: if total animal numbers have increased as a result of this amendment(s), please ensure to complete Section E of this form. AUT-F0377-5 6/9 Species Life stage Strain/breed Genetic status Genetic alteration (if GA) Breeder/supplier establishment authorisation number Country of origin* Have any individual animal(s) proposed for use in this project been previously used in a scientific study? If yes, describe the cumulative effect of the procedures on the animal(s)? Number of animals to be used Yes No * If animal(s) are sourced outside of the Republic of Ireland, please indicate the source of the animals and provide a certificate confirming authorisation and registration of the supplier establishment (as required under Directive 2010/63/EU) where animal(s) were bred / supplied in the country of origin. SECTION E: TOTAL ANIMAL NUMBERS If the amendment(s) involves an increase in animal numbers please state: 1 Total number of animals currently authorised for use: 2 Amended total number of animals: 3 Provide justification for the additional animal numbers requested, including statistical calculations if relevant/appropriate. SECTION F: DECLARATION AND UNDERTAKING The declaration and undertaking below should be signed by or on behalf of the applicant i.e. by the project manager or proposed new project manager (designated pursuant to Regulation 47 of S.I. No. 543 of 2012 as amended), who is responsible for the overall implementation of the project and its compliance with the project authorisation. I hereby declare that: - I have been designated by the user to make this application on the user’s behalf. - The information contained in this application is true and correct. I hereby undertake that in the event of the amendment or renewal of the project authorisation being granted: - To ensure fulfilment of the obligations arising by virtue of the terms and conditions of the project authorisation. - To ensure fulfilment of the requirements of S.I. No. 543 of 2012, including: AUT-F0377-5 7/9 - - - To submit an application for an amendment if any further substantial changes to the project are required. To ensure that the project manager has a valid individual authorisation. To ensure that, if appointed, the deputy project manager has a valid individual authorisation for the purpose of project management. To ensure that all persons carrying out procedures under this project have a valid individual authorisation. To ensure that all persons performing euthanasia under this project have a valid individual authorisation. To ensure the methods of euthanasia performed are in accordance with Annex IV of Directive 2010/63/EU unless an exemption is granted by the HPRA. To report any project deviations that have an adverse effect on animal health or welfare, and to report any changes to a severity classification that have an adverse effect on animal health or welfare to the designated veterinarian and/or the animal welfare body at the user establishment. To keep written records of all animals used under this project authorisation for a minimum of 3 years, and to make all written records or project documentation available to the HPRA upon request or as part of an inspection. To provide the user establishment with an end of project report for the finished project to be made available to the HPRA upon request. To fulfil all reporting requirements including annual statistical returns to the HPRA. To provide updates (if any) to the non-technical project summary (where relevant) to the HPRA, which will be made publically available by the HPRA. To comply with the requirements of S.I. No. 543 of 2012 for the care and accommodation of animals. Signature of project manager: Print/type name: Date: _________________________ COMPLIANCE OFFICER SIGNATURE (FOR ALL AMENDMENT TYPES) The declaration below should be signed by the compliance officer (designated pursuant to Regulation 44 of S.I. No. 543 of 2012 as amended) responsible for ensuring compliance with the provisions of S.I. No. 543 of 2012 at the relevant user establishment. I hereby declare that: - The applicant is affiliated to the user establishment referred to in section A. - I understand that if the applicant fails to uphold his/her responsibilities under S.I. No. 543 of 2012 in the user establishment or additional locations for which I am compliance officer, this may have implications for the continued authorisation of the user establishment. Signature of compliance officer: __________________________ (on behalf of breeder/supplier/user) Print/type name: Date: COMPLIANCE OFFICER SIGNATURE (AMENDMENT TO ADD NEW USER ESTABLISHMENT) AUT-F0377-5 8/9 The declaration below should be signed by the compliance officer (designated pursuant to Regulation 44 of S.I. No. 543 of 2012 as amended) responsible for ensuring compliance with the provisions of S.I. No. 543 of 2012 at the relevant user establishment. I hereby declare that: - The applicant is affiliated to the user establishment referred to in section D5 - I understand that if the applicant fails to uphold his/her responsibilities under S.I. No. 543 of 2012 in the user establishment or additional locations l for which I am compliance officer, this may have implications for the continued authorisation of the user establishment. Signature of compliance officer: __________________________ (on behalf of breeder/supplier/user) Print/type name: Date: CHECKLIST Amended project protocol (relevant sections only) – highlighted to identify changes Certificate confirming authorisation and registration of breeding / supplying establishment (for animals sourced outside the Republic of Ireland) Copy of ethical review application and associated documentation (where relevant) Ethics approval document (where relevant) CV (setting out education, training, experience and publication history) (where relevant) Animal welfare body recommendation (where relevant) Submit the completed form with attachments via CESP, or to sapsubmit@hpra.ie or in hard copy to: Receipts and Validation Department Health Products Regulatory Authority Kevin O’Malley House Earlsfort Centre Earlsfort Terrace Dublin 2 Tel: + 353 1 676 4971 Fax: + 353 1 676 7836 AUT-F0377-5 9/9







