223890_Semester_2_unit_review
Semester 2 unit review
The following units will be covered on the final exam.
Unit 6 (Chemical Composition), Unit 7 (Hydrates), Unit 8 (Chemical Quantities),
Unit 10 (Chemical Bonding), Unit 12 (Gases), Acids and Bases
The following concepts should be reviewed;
Unit 6 (Chemical Bonding)
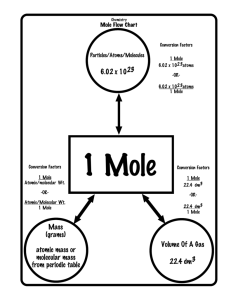
Mole conversions
What mass of carbon contains the same number of atoms as 1.008g of hydrogen?
Molar Mass
Calculate the number of moles for each of the following:
200.0 grams NaOH
Percent Composition
Calculate the percent elemental composition in Na
2
CO
3
, iron (II) chloride, barium hydride.
Moles of an element
How many moles of hydrogen in 67.6 moles of NH
4
F
Empherical and Molecular formulas
A compound with the empirical formula C2HF4 was found to have a molar mass of about 200g/mole. What is the molecular formula of the compound?
Unit 7 (Hydrates)
A hydrate is analyzed and found to have .737 g magnesium sulfite and
.763 g of water. Find the formula of this hydrate and name it.
A hydrated crystal is found to be 89.2 % barium bromide, and 10.8 % water. What is the formula and name of this hydrate? (Hint: Pretend you have 100g sample.)
Unit 8 (Chemical Quantities)
Mole conversions
For each of the following balanced reactions, calculate how many moles of each product would be produced by complete conversion of 0.50 mol of the reactant indicated in boldface. Write clearly the mole ratio used for the conversion.
2H
2
O
2
(l) 2H
2
O(l) + O
2
(g)
Mass Calculations
Although we usually think of substances as "burning" only in oxygen gas, the process of rapid oxidation to produce a flame may also take place in other strongly oxidizing gases. For example, when iron is heated and placed in pure chlorine gas, the iron "burns" according to the following
(unbalanced) reaction.
Fe(s) + Cl
2
(g) FeCl
3
(s)
How many milligrams of Iron(III) chloride result when 15.5 mg of iron is reacted with an excess of chlorine gas?
Although mixtures of hydrogen and oxygen are highly explosive, pure elemental hydrogen gas itself burns quietly in air with a pale blue flame, producing water vapor.
2H
2
(g) + O
2
(g) 2H
2
O(g)
Calculate the mass in grams of water vapor produced when 56.0 g of pure hydrogen gas burns
Limiting Reactions
If 14.4 grams of zinc metal is dropped into 125 mL of a 1.50 M solution of phosphoric acid, H
3
PO
4
. What mass of zinc phosphate and what mass of hydrogen is produced in the reaction?
The limiting reactant is __________. The reactant in excess is ______.
Percent Yield
According to her pre-lab stoichiometric calculation, a student’s lab experiment should have produced 5.51 g of NaCl. While actually doing the experiment, this student obtained only 4.32 g of NaCl. What was her percent yield?
Unit 10 (Chemical bonding)
Bonding Types
Review following terms; electronegativity, polar covalent, ionic, non-polar covalent
Lewis Structures
Write a Lewis structure for each of the following simple molecules. Show all bonding valence electron pairs as a line and all nonbonding valence electron pairs as dots. For those molecules that exhibit resonance, draw the various possible resonance forms.
a. CO b. CO
2
c. O
2 d. H
2
Se
Intermolecular Forces
Review the following terms; LDF, dipole-dipole forces, hydrogen bonds, boiling points.
Like Dissolves Like
Why does an ionic solid dissolve in water? How does this happen?
Is gasoline (lots of C-C and C-H bonds) miscible with water? Why or why not?
Is sugar soluble in water? Why or why not?
Is ethyl alcohol (ethanol) soluble in water? Why or why not?
Which of the following substances would be soluble in water and which would be soluble in gasoline?
Unit 12 (Gasses)
Boyles Law
The pressure exerted on a 240. ml sample of hydrogen gas at constant temperature is increased from 0.428 atm to 0.724 atm. What will the final volume of the sample be?
Charles Law
An expandable vessel contains 729 mL of gas at 22 o C. What volume will the gas sample in the vessel have if it is placed in a boiling water bath
(100.
o C)?
Avogadro’s Law
If 4.0 g of helium gas occupies a volume of 22.4 L at 0 o C and a pressure of 1.0 atm, what volume does 3.0 g of He occupy under the same conditions?
Ideal gas law
P=755 torr; V=?L; n=0.341mol; T=22 C
Combined Gas Law
A 350. mL air sample at 47 degrees Celsius has a pressure of 550. torr.
What pressure will the air exert of it is allowed to expand to 435 mL at
57 degrees Celsius.
Acids Bases
(Questions from Presentations)