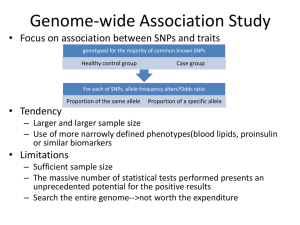
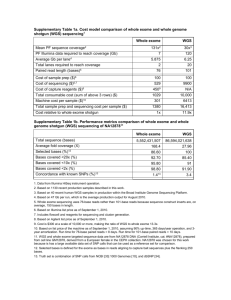
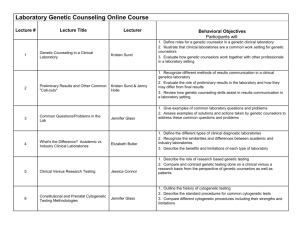
Exome Sequencing - ARUP Laboratories
advertisement

[Date] [Insurance Company] [Address 1] [Address 2] [City State Zip] Re: [Patient Name] Insurance ID: [ID #] DOB: XX/XX/XXXX To whom it may concern, I am writing on behalf of my patient, [patient’s name], to request insurance coverage for whole exome sequencing to aid in establishing a diagnosis. [Describe relevant clinical findings/family history] I have given serious consideration towards this patient’s underlying diagnosis and while I think that it is highly likely that [he/she] has a genetic syndrome, at the present time I cannot recognize a specific entity. Chromosomal microarray and [list other specific testing that has been performed] have not yielded a diagnosis. At this point, rather than sequencing each potentially causative gene individually, exome sequencing is the fastest and most cost-effective way to assess for a genetic etiology amongst a large number of genes. Recent studies have supported exome sequencing as a method to identify genes/diseases that were not recognized using traditional single gene tests or gene panels1. Whole exome sequencing is expected to provide a definitive diagnosis in 25% of individuals with a suspected genetic disorder in which routine genetic testing has not identified the cause2. Exome sequencing has been used in a clinical setting to find the molecular causes of disease including intractable bowel disease1, mental retardation2 and ataxia3, among many others. Results from this genetic test will have a direct impact on this patient’s treatment and will provide prognostic information that will assist in clinical management. Additionally, if mutation(s) are identified using exome sequencing, such information would be important for accurate genetic counseling regarding recurrence risk. Whole exome sequencing is necessary for my patient to determine the nature of [his/her] condition and identify appropriate medical management and therapies in a timely fashion. In the absence of this testing, I will not be able to provide a diagnosis or appropriate management recommendations for this patient’s care. Please note that this test is performed for clinical management in a laboratory that is CLIA approved for high complexity testing. Please take these factors into consideration and provide the coverage for the whole exome sequencing test that I am recommending for this patient. Authorization should be obtained for: Test name: Exome Sequencing Symptom-Guided Analysis, Patient Only Facility: ARUP Laboratories CPT codes: [CPT codes] Diagnosis code: [Diagnosis code] Thank you for your time and attention to this matter. Sincerely, [Name of Ordering Physician] Reference: 1. Worthey, EA et al. Making a definitive diagnosis: successful clinical application of whole exome sequencing in a child with intractable inflammatory bowel disease. Genet Med 2011. 13(3):255262. 2. YangYaping, Muzny Donna, et. al. Clinical Whole-Exome Sequencing for the Diagnosis of Mendelian Disorders. NEJM 2013 Oct pp1-10. 3. Vissers, LE et al. A de novo paradigm for mental retardation. Nat Genet 2010. 42(12):1109-1112. 4. Liew WK, et al. Clinical application of whole-exome sequencing: a novel autosomal recessive spastic ataxia of Charlevoix-Saguenay sequence variation in a child with ataxia. JAMA Neurol 2013. 70(6):788-791.