Suppl. Material
advertisement

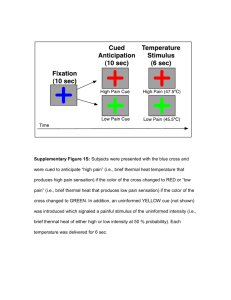
Contents: 1. Supplementary Methods a. Prolonged Exposure Therapy [page 1] b. Psychological Measures [page 1] c. Neuropsychological Measures [page 2] d. Stimulus and Apparatus [page 2] e. Image Acquisition [page 2] f. Functional Imaging Analysis [page 3] g. Functional connectivity analysis [page 4] 2. Supplementary Results a. Brain x Behavior [page 6] 3. Supplementary Tables and Figures [page 7] 4. References [page 11] 1. Supplementary Methods Prolonged Exposure Therapy: During PE, patients complete twelve 90-min individual treatment sessions. PE has three main components 1) psycho-education and deep breathing, 2) in-vivo exposure, and 3) imaginal exposure. Psycho-education involves providing information on general PTSD aspects and planning a course of treatment. The goal of this phase is to help patients understand that people often have similar experiences after a life-threatening combat experience and to allow them to be prepared to engage in a challenging but efficacious intervention. Deep breathing involves training in a core technique for physiological control and relaxation that can aid in the processes of working through exposure experiences. In-vivo exposure involves creating a hierarchy of situations that patients fear and avoid and then gradually exposing them to these situations to help patients habituate to them. Patients create the hierarchy during the sessions and practice daily in between sessions, to perpetuate habituation. Imaginal exposure involves having patients review the traumatic event that caused the PTSD in session by mentally placing themselves back in the situation. Patients are instructed to close their eyes and 1 speak in the present tense while undergoing imaginal exposure and listen to audio recordings of the imaginal exposures daily in between sessions. Psychological Measures: PTSD diagnosis was determined by the Clinically Administered PTSD scale(CAPS)(1), a structured interview with 17 items from the DSM-IV criteria B, C, and D. The Structured Clinical Interview for DSMIV Axis I Disorders(SCID)(2), without the PTSD module, was used to diagnose major Axis I disorders, including depression and substance abuse/dependence. The Deployment Risk and Resilience Inventory(DDRI)(3) was administered to determine relevant risk factors pre, during, and post trauma. The DDRI is a collection of measures for studying deployment-related experiences of military personnel and veterans validated on military serving in the Gulf War. In addition, all subjects completed the Connor Davidson Resilience Scale(CD-RISC-10)(4) and the Toronto Alexithymia Scale(TAS)(5) to quantify resilience and the ability to identify and describe emotions, respectively. Clinical interview occurred on the same day as the scanning session and was conducted by trained staff and validated by a clinical psychologist (ANS). Neuropsychological Measures: Neuropsychological battery, which took place immediately following the end of second imaging session, consisted of the following tests covering a variety of cognitive domains with particular emphasis on memory, executive function, and attention: Wechsler Adult Intelligence Scale-3rd Edition(WAIS-III) subtests(6), Vocabulary, Letter Number Sequencing, Matrix Reasoning, Controlled Oral Word Association Test(FAS)(7), DKEFS Stroop Color and Word Test(8), and The Trail Making Test(TMT), Parts A and B(9). Stimulus and Apparatus: 2 During fMRI, all subjects performed a cued anticipation task that combined a continuous performance task(CPT) with the interspersed presentation of aversive affective stimuli(supplementary Figure 1). During the CPT, subjects were instructed to press a LEFT mouse button whenever they saw a blue circle and a RIGHT mouse button whenever they saw a blue square on the screen. Stimuli were presented at a visual angle of 4 degrees at a rate of 0.5Hz. Simultaneously, a 250msec long 500Hz tone was presented at a rate of 2Hz. Subjects were instructed prior to the task that a switch to a green shape accompanied by a 250Hz tone would indicate that a positive image was going to appear, and that a switch to a red shape accompanied by a 1000Hz tone would indicate that a negative image was going to appear. Seventeen positive images were taken from the International Affective Picture System(IAPS). Seventeen negative images were selected that displayed individuals in combat situations without graphic violence or open wounds. The total duration of the task was 580 seconds. Behavioral data related to performance of the CPT were collected and scored for accuracy and latency of response. Response accuracy and response latency(RT) were obtained during:(1) performance of the CPT,(2) anticipation of a positive image(PA), and(3) anticipation of a negative image(NA). Image Acquisition: During the task, an fMRI run sensitive to BOLD contrast was collected for each subject using a Signa EXCITE(GE Healthcare, Milwaukee) 3.0T scanner(T2*weighted echo planar imaging, TR=2000ms, TE=32ms, FOV=250x250mm3, 64×64 matrix, 30 2.6mm axial slices with a 1.4mm gap, 290 scans). fMRI acquisitions were time-locked to the onset of each trial. During the same experimental session, a highresolution T1-weighted image(TI=450ms, TR=8ms, TE=4ms, flip angle=12°, FOV=250x250, 172 sagittal slices, ~1mm3 voxels) was obtained for anatomical reference. Functional Imaging Analysis: 3 Functional data were preprocessed and analyzed with the Analysis of Functional NeuroImages(AFNI) software package. Preprocessed time series data for each individual were analyzed using a multiple regression model. Regressors of interest included: 1) the PA, i.e. what activated during anticipation of a positive image and 2) the NA, i.e. what activated during anticipation of a negative image. In addition, nuisance regressors were entered into the linear regression model: positive and negative image phases, three movement-related regressors used to account for residual motion(in the roll, pitch, and yaw direction), and regressors for baseline and linear trends used to eliminate slow signal drifts. To reduce the false positives induced by cross correlations of the time series data was fit using the AFNI program 3dREML. Subsequently, contrasts were constructed on an individual subject level for differential anticipation of negative and positive(i.e. NA- PA) images. A Gaussian filter with full widthhalf maximum 6 mm was applied to the voxel-wise percent signal change data to account for individual variations in the anatomical landmarks. Data of each subject were normalized to Talairach coordinates as defined by AFNI’s built-in atlases. Voxel-wise percent signal change data were entered into a linear mixed effects model with Time(pre/post-therapy) and Group(remitted/non-remitted, i.e., R-PTSD/NR-PTSD) as fixed factors and subjects entered as a random factor. Analysis was done with the AFNI function 3dLME.R which used the statistical program R(www.cran.org) and the lm library. Results are displayed that differ significantly in the interaction(GroupxTime), between remitting(R-PTSD) and non-remitting(NR-PTSD; Group effect), and over time(pre/post; Time effect). These results were masked by those that showed a significant task effect for the NA-PA contrast in both groups. A Monte-Carlo simulation(iterations =10,000) using AlphaSim was used to determine that a cluster size of 832mm3(13voxels) in the task-related areas was required to control for multiple comparisons maintaining an alpha of .05. The cluster F-values were calculated by averaging the voxel 4 based F-values in each cluster. Average percent signal difference was extracted for post-hoc correlational analysis. Functional connectivity analysis: Functional connectivity analysis was performed on both R-PTSD and NR-PTSD groups. The connectivity results are reported for the NA–PA contrast. We used functional connectivity method based on the psychophysiological interaction method introduced by Friston et al.(1997) and adapted for AFNI(http://afni.nimh.nih.gov/sscc/gangc/CD-CorrAna.html). Before conducting the functional connectivity analysis the individual raw signal datasets were: 1) band-pass filtered(0.009<ƒ<0.08), 2) corrected for slice-dependent time shifts, 3) corrected for interleaved acquisition, 4) corrected for rigid body head motion, and 5) warped to conform to the Talairach atlas(23). Individual time courses in these processed raw signal datasets were extracted for the seed ROI, namely left ventral anterior insula that showed significant TimexGroup interaction(Figure 1). Data points were censored if they differed by more than 2 standard deviation from the average echoplanar signal for the seed ROI. We then used AFNI function 3dTfitter to remove hemodynamic delay from the insula timecourse. The resultant signal was then multiplied by the differential anticipation regressor(i.e, NA-PA) thereby creating an interaction timecourse, which was then convolved with gamma-variate hemodynamic using the AFNI function “waver.” Interaction time course were used as regressor of interest together with nuisance regressors controlling for all task regressors, baseline differences, linear drift, non-time interaction seed time series, and head movement(roll, pitch, and yaw). REML fitting was performed to reduce false positives due to time-dependent cross correlations. The resulting correlation coefficient for the timecourse in the insula ROI was calculated for each voxel. This provided correlation maps for the time course in the insula ROI and the time course from all other brain voxels as a function of differential anticipation. The Fisher Z transforms of these correlation maps were then warped to conform to the Talairach atlas(23) and a 5 Gaussian blur of 6 mm FWHM was applied. Voxel-wise data were entered into a linear mixed effects model with Time(pre/post-therapy) and Group(R-PTSD/NR-PTSD) entered as fixed factors and subjects entered as a random factor. Results are displayed that differed significantly in the interaction(GroupxTime), between remitting(R-PTSD) and non-remitting(NR-PTSD; Group effect), and over time(pre/post; Time effect). The resulting data was cluster thresholded using Monte-Carlo simulations as described above, resulting in a minimum cluster of 3072mm3(48voxels) in the whole brain. 2. Supplementary Results: Brain x Behavior Relationships: To investigate how signal change(anterior insula) or strength of connectivity changes between pre and post-treatment(cingulate, mid-posterior insula), related to psychological and neuropsychological variables we performed exploratory post-hoc Pearson correlations between the pre/post signal change and behavioral/neuropsychological measures. Within the group as a whole(i.e., R-PTSD and NR-PTSD combined) only the WAIS vocabulary(r=.457, n=24, p=0.033), CD-RISC-10(r=.614, n=24, p=0.007), and TAS-20: Difficulty Describing Feelings(r=-.564, n=24, p=0.015) correlated significantly with the change in functional connectivity between the anterior insula and right cingulate. Within the non-remitting group a similar correlation was found with the WAIS Vocabulary(r=.589, n=24, p=0.021), CD-RISC-10(r=.701, n=24, p=0.011), and TAS-20: Difficulty Describing Feelings(r=-.765, n=24, p=0.004) scores. However, several weak correlations existed between change in the mid-posterior insula connectivity with Trails A time(r=.525, n=24, p=0.045) and the change in anterior insula with Stroop Color Naming(r=-.545, n=24, p=0.044). In the remitting group, there was a correlation between change in insula activation and Trails B time(r=.761, n=24, p=0.047). 6 7 3. Supplementary Tables and Figures: Supplementary Table 1. , Group Psychological and Neuropsychological Performance Variable Remitting CI Non-Remitting CI Withdrawn Number(All Males) 9 15 Neuropsychological Battery Vocabulary SS 12.2(2.9) 10-14.4 11.7(3.1) 10-13.4 1 Matrix Reasoning SS 11.8(2.9) 9.6-14 11.5(3.3) 9.7-13.3 Letter Number Sequencing SS1 10(3.6) 7.2-12.8 9.9(2.1) 8.7-11.1 Trails A time(sec) 26.4(6.7) 21.3-31.6 26.1(9.4) 20.9-31.3 Trails B time(sec) 68.9(18.8) 54.5-83.4 61.8(20.4) 50.5-73.1 Stroop –Color Naming(sec)1 31(4.9) 27.2-34.8 29.5(6.6) 25.9-33.2 1 Stroop –Word Reading(sec) 22.2(3.6) 19.4-25 21.9(3.7) 19.9-24 Stroop –Color X Word(sec)1 56(13) 46-66 50.2(9.6) 44.9-55.5 Stroop –Color X Word Reversal(sec) 1 65.9(21.5) 49.4-82.4 52.2(9.2) 47.1-57.3 1 FAS Total Correct 45.1(10.8) 36.8-53.4 40.9(13.3) 33.5-48.3 Psychiatric Measures2 CD-RISC 10 31.4(5) 27.6-35.2 21.5(10.6) 15.6-27.4 TAS-20:Difficulty Identifying Feelings 20(9.7) 12.5-27.5 24.2(5) 21.4-27 TAS-20:Difficulty Describing Feelings 17.8(3.7) 15-20.6 18.8(3.5) 16.9-20.7 TAS-20:Externally-Oriented Thinking 21.8(3.6) 19-24.6 26.3(2.4) 25-27.6 TAS-20:Total 59.5(11.8) 50.4-68.6 69.2(8.4) 64.6-73.9 DRRI:Childhood Stress 4.1(2.8) 2-6.3 4.7(2.9) 3.1-6.3 DRRI:Prior Stress 43.4(5.4) 39.3-47.6 43.6(4.5) 41.1-46.1 DRRI:Combat Exposure Stress 55.2(11.9) 46.1-64.4 47(9.8) 41.6-52.4 DRRI:Life Disruption 58.1(9.1) 51.1-65.1 59.4(8.7) 54.6-64.2 DRRI:Social Support 24.4(9.7) 16.9-31.9 25.7(13.4) 18.3-33.1 DRRI:Work Environment 44.4(13.8) 33.8-55 40.7(17.1) 31.2-50.2 DRRI:Post Battle Exposure 18.7(4.5) 15.2-22.2 22.9(7.5) 18.8-27.1 DRRI:Harassment 51.3(5.3) 47.2-55.4 51.9(8.8) 47-56.8 DRRI:Perceived Threat 8.9(3.6) 6.1-11.7 10.5(3.4) 8.6-12.4 DRRI:Toxic Exposure 12.4(3.5) 9.7-15.1 12(3.3) 10.2-13.8 DRRI:Preparedness 23.2(6.2) 18.4-28 22.9(7.2) 18.9-26.9 DRRI:Post Stressors 53.2(15.4) 41.4-65 56.9(6.8) 53.1-60.7 DRRI:Post Social Support 5(3.8) 2.1-7.9 5.5(3.2) 3.7-7.3 1 2 Note. =One Non-remitting subject did not complete test item; = Non-remitting subjects(n=10) and remitting subjects(n=8 or 7 for DRRI) ; SS=Scaled Score; sec=seconds; Supplementary Table 2. Whole brain Treatment (R-PTSD/NR-PTSD) by Time (Pre/Post) Effects voxels x y z F-stat Within BA 314 -6 -72 -15 8.025 Left Cerebellum 280 42 -17 19 7.568 Right Insula 13 100 14 23 40 6.287 Right Medial Frontal Gyrus 32 86 -31 -45 22 6.008 Left Insula 13 75 -43 28 6 7.422 Left Insula/Inferior Frontal Gyrus 13 8 75 74 71 68 54 45 42 -25 -14 18 15 19 -23 -35 -15 41 7.348 -46 -27 8.427 37 1 6.259 -35 14 7.564 -86 2 10.179 14 30 6.166 -43 -18 7.013 Left Middle Frontal Gyrus Left Cerebellum Right Anterior Cingulate Right Thalamus Right Lingual Gyrus Left Cingulate Gyrus Left Fusiform Gyrus 9 24 32 17 32 20 Supplementary Figure 1. Affective anticipation task. In this task, blue shapes are presented at baseline, while green shapes cue positive images and red shapes cue negative images. 10 Supplementary Figure 2. Whole brain Treatment (R-PTSD/NR-PTSD) by Time (Pre/Post) Effects 11 12 4. References 1. Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, et al. The development of a Clinician-Administered PTSD Scale. JTrauma Stress. 1995;8(1):75-90. 2. First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Non-patient Edition. (SCID-I/NP). New York Biometrics Research, New York State Psychiatric Institute; 2002. 3. King LA, King DW, Vogt DS, Knight J, Samper RE. Deployment risk and resilience inventory: A collection of measures for studying deployment-related experiences of military personnel and veterans. Military Psychology. 2006;18(2):89-120. 4. Campbell-Sills L, Stein MB. Psychometric analysis and refinement of the Connor-davidson Resilience Scale (CD-RISC): Validation of a 10-item measure of resilience. Journal of traumatic stress. 2007;20(6):1019-28. 5. Bagby RM, Parker JDA, Taylor GJ. The twenty-item Toronto Alexithymia Scale--I. Item selection and cross-validation of the factor structure. Journal of psychosomatic research. 1994;38(1):23-32. 6. Wechsler D. Wechsler Memory Scale - Third Edition. Texas Psychological Corporation; 1997. 7. Benton AL. Development of a multilingual aphasia battery. Progress and problems. Journal of the neurological sciences. 1969;9(1):39-48. 8. Delis DC, Kaplan E, Kramer JH. Delis-Kaplan executive function system: The Psychological Corporation; 2001. 9. Reitan RM. The relation of the Trail Making Test to organic brain damage. Journal of Consulting Psychology. 1955;19(5):393. 13