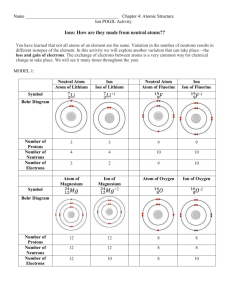
Chapter 3 Study Guide “Elements, Atoms, and Ions” What were did
advertisement

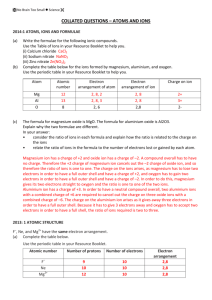
Chapter 3 Study Guide “Elements, Atoms, and Ions” 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. 14. 15. What were did some of the early “chemists” or alchemists try to create? What are elements? How many elements are presently known? What are 3 things that elements are named after? Provide a list of elements whose names begin with the letter C. List the 7 diatomic molecules. What are ions? Who discovered the Plum Pudding model? Draw the model. Explain what Rutherford’s gold foil experiment proved. James Chadwick discovered which particle? How is a photon, or light particle, produced? Provide the charge and the locations of each of the subatomic particles. AZX notation is used for what? What is an anion and how is it formed? What is a cation and how is it made? Fill in the chart below. Element X exists in three forms – a neutral atom, an isotope, and an ion. What do these three forms have in common and what do they not have in common? Chapter 3 Study Guide “Elements, Atoms, and Ions” 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. 14. 15. What were did some of the early “chemists” or alchemists try to create? What are elements? How many elements are presently known? What are 3 things that elements are named after? Provide a list of elements whose names begin with the letter C. List the 7 diatomic molecules. What are ions? Who discovered the Plum Pudding model? Draw the model. Explain what Rutherford’s gold foil experiment proved. James Chadwick discovered which particle? How is a photon, or light particle, produced? Provide the charge and the locations of each of the subatomic particles. AZX notation is used for what? What is an anion and how is it formed? What is a cation and how is it made? Fill in the chart below. Element X exists in three forms – a neutral atom, an isotope, and an ion. What do these three forms have in common and what do they not have in common? Chpt 3 Study Guide Answer Key 1. What was the primary goal of alchemists? a. Turn metals into gold 2. What are elements? How many elements are presently known? a. An element is a pure substance that cannot be chemically broken down into a simpler form of matter (unlike compounds). There are currently 118 elements, 92 of which are naturally occurring. 3. What are 3 things that elements are named after? Many of the elements have symbols that do not match their name. Why is this? a. People, countries, properties b. The symbols are based on the element’s original Greek, Latin name 4. Provide a list of elements whose names begin with the letter C. a. calcium (Ca), cobalt (Co), carbon (C), chlorine (Cl), chromium (Cr), cesium (Cs), cerium (Ce), californium (Cf), cadmimum (Cd) 5. List the 7 diatomic molecules. a. hydrogen – H2, nitrogen – N2, oxygen – O2, fluorine – F2, chlorine – Cl, bromine – Br2, iodine – I2 6. What are ions? a. Ions are atoms that contain a charge. 7. Who discovered the Plum Pudding model? Draw and label the model. a. J.J. Thomson 8. Explain what Rutherford’s gold foil experiment proved. a. Rutherford’s gold foil experiment proved that atoms contained a center of positive charge (nucleus filled with protons). The rest of the atom was made of empty space where the electrons are located. 9. James Chadwick discovered which particle? Neutron 10. How is a photon, or light particle, produced? a. A photon is produced when an electron falls down from the excited state (higher energy) back to the ground state (lower energy). An atom is excited when it is exposed to a form of high energy (heat, electricity, light) 11. Provide the charge and the locations of each of the subatomic particles. a. protons – positive charge, nucleus b. neutrons – neutral, nucleus c. electron – negative, orbits 12. AZX notation is used for what? AZX notation is used to distinguish between isotopes of an element 13. What is an anion and how is it formed? What is a cation and how is it made? a. A cation is positive ion due to the lose of electrons b. An anion is a negative ion due to the gain of electrons 14. Fill in the chart below. Element Symbol Atomic Number Osmium Os 76 Lithium Li+ 3 40 Calcium 20 20Ca 41 Calcium Ca 20 20 Fluorine F 9 Mass Number 190 7 40 41 19 Protons Neutrons Electrons 76 3 20 20 9 114 4 20 21 10 76 2 20 20 10 15. Element X exists in three forms – a neutral atom, an isotope, and an ion. What do these three forms have in common and what do they not have in common? a. A neutral atom has the same number of protons and electrons. An isotope has the same atomic number (protons), but a different mass number due to a different number of neutrons. An ion has a more or less electrons.