Lesson 22 Microscopic magnetic moments Motion of charged
advertisement
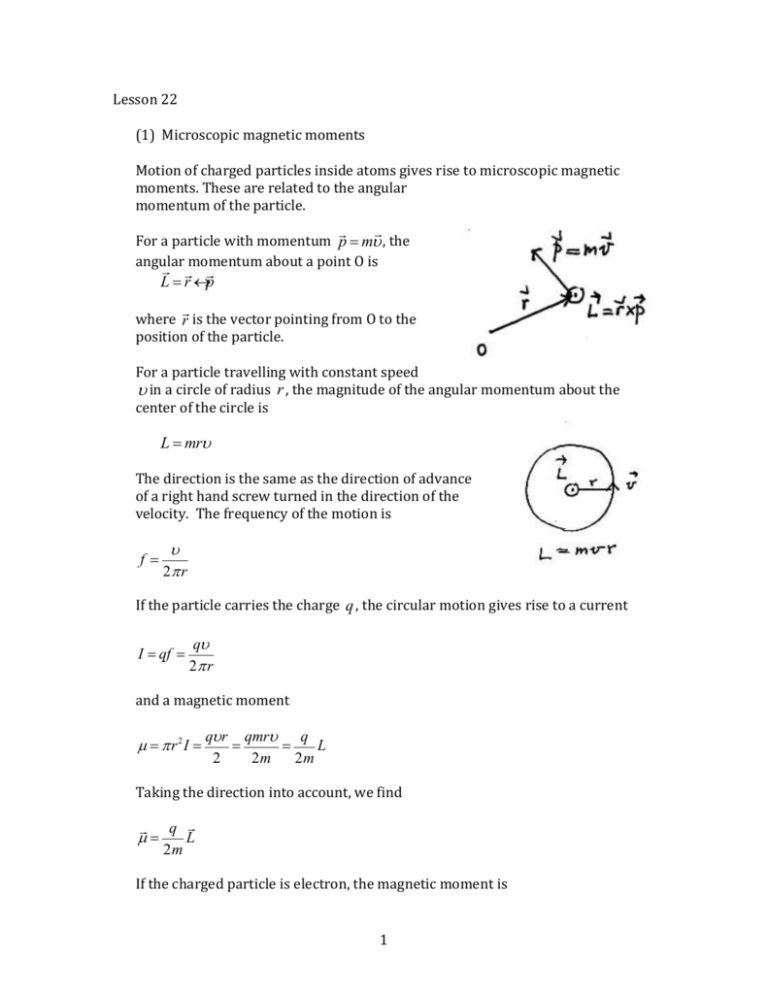
Lesson 22 (1) Microscopic magnetic moments Motion of charged particles inside atoms gives rise to microscopic magnetic moments. These are related to the angular momentum of the particle. For a particle with momentum p = mu , the angular momentum about a point O is L=r´p where r is the vector pointing from O to the position of the particle. For a particle travelling with constant speed u in a circle of radius r , the magnitude of the angular momentum about the center of the circle is L = mru The direction is the same as the direction of advance of a right hand screw turned in the direction of the velocity. The frequency of the motion is f= u 2p r If the particle carries the charge q , the circular motion gives rise to a current I = qf = qu 2p r and a magnetic moment m = p r2I = qu r qmru q = = L 2 2m 2m Taking the direction into account, we find m= q L 2m If the charged particle is electron, the magnetic moment is 1 mL = - e L 2me In quantum mechanics, the angular momentum is found to be an integral multiple of Planck constant , which has the value = h =1.05´10-34 J × s 2p Expressing angular momentum is units of m L = -m B , we can write L after introducing the Bohr magneton mB = e = 9.27 ´10-24 A × m 2 = 9.27 ´10-24 J / T = 5.79 ´10-5 eV / T 2me The Bohr magneton gives an estimate of the size of microscopic magnetic moment due to electrons. An electron also has angular momentum due to its intrinsic spin that is unrelated to motion. The spin angular momentum S gives rise to magnetic moment mS = -2 e S S = -2m B 2me Although the factor for m S is twice that for m L , the spin angular momentum S assumes half-integral values instead of integral values as for orbital angular momentum L . (2) Magnetization By definition, the magnetization of a material is the magnetic moment in unit volume of the material. The magnetic moment is the vector sum of the atomic magnetic moments. In most substances, the atomic magnetic moments are randomly orientated, so that the magnetization is zero. If all atomic magnetic moments are aligned, we find the maximum magnetization called saturation magnetization. 2 Example: Find the saturation magnetization of iron, assuming that each atom has the magnetic moment of 1 Bohr magneton. Solution: Density of iron = 7.87´103 kg / m3 Molar mass of iron = 55.8g / mole = 55.8´10-3 kg / mole Number density of atoms 7.87 ´103 kg / m3 n= ´ 6.02 ´10 23 atoms / mole = 8.49 ´10 28 atoms / m3 -3 55.8´10 kg / mole Saturation magnetization M sat = nmB = 8.49 ´1028 atoms / m3 ´ 9.27´10-24 A× m = 7.88´105 A / m Magnetization of a material is associated with macroscopic current and magnetic field. To find the current, consider a slab of material with area A and thickness L . Consider the atomic currents giving rise to the atomic moments to be square loops of current i of area a , filling up the whole area A , and that there are N L layers of such loops in the thickness L . Number of loops in a layer N A = A a Magnetization = åm = N V N Aia N Li = LA L L Adding up these loop currents, we see that there is no current in the interior, but there is a current I = N Li running on the edges of the slab called the Amperean current. This behaves like a solenoid current and creates a magnetic field in the interior equal to BM = m0 NL I i = m0 = m0 M L L Example: For the sample of iron in the previous example, the magnetic field due to maximum magnetization is B = m0 M sat = 4p ´10-7 ´ 7.88´105 = 0.99T (3) Induced magnetization 3 The magnetization of most substances is zero. It can become non-zero when an external magnetic field Bapp is applied, such as by placing the sample inside a solenoid. This is called induced magnetization. For paramagnetic and diamagnetic substances, the induced magnetization is proportional to the applied field, so that we can write M = cm Bapp m0 where the material-dependent constant c m is called magnetic susceptibility. It is positive for a paramagnetic substance and negative for a diamagnetic substance. Since the total magnetic field inside the sample is B = Bapp + m0 M = (1+ c m ) Bapp it is enhanced in a paramagnetic substance and reduced in a diamagnetic substance. The values of susceptibility for most substances are quite small. When an inhomogeneous external magnetic field is applied to the sample, it is attracted to the region of strong field is it is paramagnetic, and expelled from that region if it is diamagnetic. This can be explained by the fact that the induced magnetization is parallel to the applied field for paramagnetic and anti-parallel fro diamagnetic materials, if we also remember that a magnetic moment behaves like a small bar magnet. The explanation is illustrated in the diagrams shown. However, the forces involved are weak. (4) Paramagnetism In a paramagnetic substance, the atoms have permanent magnetic moments. Because of thermal motion, these magnetic moments are randomly orientated. They are aligned to some degree when an external magnetic field is applied, 4 giving rise to magnetization. We can estimate this magnetization in the following manner. Magnetic energy of atom ~ matom Bapp Random thermal energy of atom ~ kT where T = temperature in K, k = Boltzmanncons tant =1.38´10-23 J / K Since the applied field counteract randomization due to thermal motion, the fraction of magnetization versus saturation magnetization can be estimated to be m B m B M M ~ atom app , with = atom app in an exact calculation M sat kT M sat 3kT This leads to the magnetic susceptibility cm = matomm0 M sat 3kT which is known as Curie’s law. According to this, susceptibility decreases with temperature. As an example, aluminum is paramagnetic, with susceptibility c m = 2.2 ´10-5 at 20 C . (5) Ferromagnetism In iron, cobalt, and a few other metals, because of the strong forces between the electron spins in neighboring atoms, the spins are lined up in the same direction, making large magnetic moments. These magnetic moments exist in domains, but the directions of the magnetic moment in the domains are still random, so that magnetization is still zero ordinarily. 5 When a magnetic field is applied, the domains can slip against each other, causing their magnetic moments be aligned with the applied field. Much larger induced magnetization occurs compared with paramagnetic substances. The substance is called ferromagnetic. As the applied field increases, the magnetization also increases, proportionately to the applied field when the latter is small, but eventually more slowly and approaches saturation, as shown in a plot of M against Bapp . If the applied field is now reduced, so does the magnetization. However, the curve of M against Bapp does not retrace the original path. In particular, when the applied field is reduced to zero, magnetization does mot go back to zero, and takes on a finite value. The material now becomes a permanent magnet. Increasing the applied field in the opposite direction can eventually reduce the magnetization to zero, (demagnetization) and further increase leads to saturation in the opposite direction. Increasing Bapp back in the original direction traces out a lower branch of the plot of M against Bapp . Thus, magnetization does not depend simply on the applied field. It also depends on which branch of the curve of M against Bapp , and therefore past history the sample has gone through. Such phenomenon is known as hysteresis. When the substance is brought through a hysteresis loop, work is done by the applied field, which is turned into heat. The work is proportional to the area inside the hysteresis loop. Above a temperature known as Curie temperature, the substance ceases to be ferromagnetic. For iron, this temperature is 770°C. We can still define susceptibility for a ferromagnetic substance by cm = m0 M Bapp except that c m now depends on Bapp and past history. If the substance is placed inside a solenoid with turn density n and current I , so that the applied field is Bapp = m0 nI ,, the magnetic field inside the substance is B = Bapp + m0 M = (1+ c m ) Bapp = (1+ c m ) m0 nI 6 m = (1+ c m ) m0 Defining magnetic permeability by B = mnI we can write the magnetic field inside the solenoid as Because of the similarity of this to the vacuum case, m 0 is called the permeability of free space. The relative permeability m m0 can be as large a few thousands, enabling the construction of strong electromagnets. (6) Diamagnetism If a substance contains free charged particles, they will describe circular motion when a magnetic field is applied. The circular motion generates magnetic moment. Considerations show that the magnetic moment is opposite to the direction of the applied field, whatever the sign of the charge. The susceptibility is then negative, and the substance is called diamagnetic. An example of such substance is high temperature plasmas, which are ionized gases made up of free electrons and positive ions. In most materials, many electrons are bound to individual atoms, travelling around the nucleus in closed orbits. Because of their random orientation, the magnetic moments due to these orbits cancel out, and no magnetization exists. When a magnetic field is applied, these orbits undergo precessions, all in the same direction. The magnetic moments associated with precessions now add up to give non-zero magnetization. The direction of this magnetization can be shown to be opposite to the applied field. The material then exhibits diamagnetism. The mechanism of diamagnetism is present in all substances. Since accordingly to Curie’s law, the magnetic susceptibility of paramagnetic substances goes down as temperature increases, all substance should become diamagnetic at high enough temperature. (7) Superconductor A superconductor not only has zero electrical resistivity, it is also a perfectly diamagnetic substance. The magnetic susceptibility is c m = -1. Therefore when it is placed in an external magnetic field, the latter cannot penetrate. The field lines near a superconductor show such exclusion. There is surface current on the conductor that generates its own field in the interior to cancel out the external field . This is called the Meisner effect. 7