Figure SI6. - Springer Static Content Server
advertisement

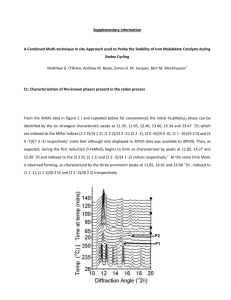
SUPPLEMENTARY INFORMATION Title: Sintered NiOx films on ITO substrates as photoactive electrodes for p-type dye-sensitized solar cells Authors: Muhammad Awais, Denis D. Dowling, Mahfujur Rahman, Han Vos, Franco Decker, Danilo Dini In the stabilized cyclic voltammogram of ITO (after 1000 cycles) the broad cathodic wave has quasi reversible character being accompanied by the two reverse oxidation processes at 1.88 V (narrower oxidation peak) and 2.15 V vs Li+/Li (broader oxidation peak) in the anodic scan (Figure SI1). From these findings it results that stabilization of the electrochemical behavior of Li y-ITO is associated with an oxide state in which the two distinct reduction processes occur approximately at the same potential. It cannot be excluded that electrochemical cycling produces an effect of grains separation with the opening of inter-grain channels due to the surface stress generated by superficial charge retention. After stabilization the scan rate (v) dependence of the current peaks has been analyzed for lithiated ITO (Figure SI2) in the range 5 ≤ v ≤ 80 mV s-1. The linearity between current peaks value and scan rate indicates the confinement of both redox processes on the surface of electroactive Li yITO layer. The dependence is linear over the whole range of scan rate used. -5 J / A cm -2 2,0x10 0,0 -5 -2,0x10 -5 -4,0x10 1,2 1,4 1,6 1,8 2,0 2,2 2,4 2,6 + E / V vs Li /Li Figure SI1. Stabilized cyclic voltammogram of ITO after 1000 cycles within the potential range 1.2-2.8 V vs Li+/Li. Cell configuration is the same as in Figure 1 (scan rate: 7 mV s-1). -4 J / A cm -2 1,0x10 0,0 -1 7mV s -1 15mV s -1 25mV s -1 40mV s -1 60mV s -1 80mV s -4 -1,0x10 -4 -2,0x10 1,2 1,4 1,6 1,8 2,0 2,2 2,4 2,6 + E / V vs Li /Li Figure SI2. Scan rate dependence of the cyclic voltammogram of uncoated ITO after 1000 cycles of stabilization (Figure SI1) within the potential range 1.2-2.8 V vs Li+/Li. Cell configuration is the same as in Figures 3and 4 (see text). -4 Peak current / A cm -2 2,0x10 -4 1,0x10 0,0 -4 -1,0x10 -4 -2,0x10 Oxidation peak at 1.9 V Oxidation peak at 2.1 V Reduction peak at 1.5 V -4 -3,0x10 0 10 20 30 40 50 v / mV s 60 70 80 90 -1 Figure SI3. Scan rate dependence of the current peaks defined according to the stabilized voltammogram of uncoated ITO (Figure SI1 and SI2). 0,0 -5 Jcp / A cm -2 -1,0x10 -5 -2,0x10 -5 -3,0x10 -5 -4,0x10 0 10 20 30 40 v / mV s 50 60 70 -1 Figure SI4. Scan rate dependence of the cathodic peak of NiOx centered at around 3.1 V vs Li+/Li (potential determined from the voltammogram at 5 mV s-1, black curve of Figure 6, see text). Dotted line represent the linear fit. -1 5 mV s -1 4,0x10 -5 15 mV s -1 25 mV s -1 40 mV s J / A cm -2 -1 55 mV s 0,0 -4,0x10 -5 2,4 2,6 2,8 3,0 3,2 3,4 3,6 + E / V vs Li /Li Figure SI5. Scan rate dependence of the cyclic voltammogram of ERYsensitized NiOx coating in dark conditions within the potential range of NiOx oxidation (Figures 5 and 7). Electrolyte composition as in Figure 5. Arrows indicate the direction of potential scan. Prior to sensitization the NiOx coating thickness was 0.3 m. -1 5 mV s 5,0x10 -1 25 mV s -5 -1 40 mV s -1 J / A cm -2 55 mV s 0,0 with illumination -5,0x10 -5 2,4 2,6 2,8 3,0 3,2 3,4 3,6 + E / V vs Li /Li Figure SI6. Scan rate dependence of the cyclic voltammogram of ERYsensitized NiOx under illumination with white light (Iin = 25 W cm-2) within the potential range of NiOx oxidation (Figures 5 and 7, see text). Electrolyte composition as in Figure 5. Arrows indicate the direction of potential scan. NiOx sample as in Figure 7 (see text). 0,0 -5 Jcp / A cm -2 -1,0x10 -5 -2,0x10 -5 -3,0x10 -5 -4,0x10 dark illuminated 0 10 20 30 v / mV s 40 50 60 -1 Figure SI7. Scan rate (v) dependence of the cathodic current density peak (JCP) in the voltammograms of ERY-sensitised NiOx coating deposited on ITO. Data have been recorded in the dark (from Figure SI5) and under illumination (from Figure SI6).