Problem SM.2 Questions
advertisement

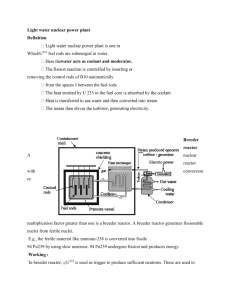
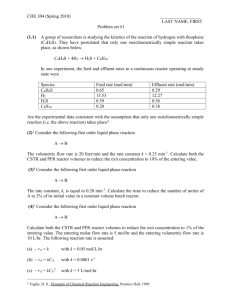
Problem SM.2 Questions your_name date 1. Based on physical properties such as critical temperature, critical pressure, and normal boiling temperature for each reaction component, why should the effluent leaving the reactor be only in the vapor or gas phase? To verify the stream phase, it may help to think of a phase (PT) diagram for each pure component. Physical properties of various chemical components are given in Ch. 1. Compound TNBP, °C TC, °C PC, kPa -252.60 64.65 100.00 110.65 136.20 145.16 -230.86 239.45 374.15 318.65 343.95 362.85 1925.55 7376.45 22,120.00 4100.04 3607.12 3840.00 Hydrogen Methanol Water Toluene Styrene Monomer Ethylbenzene Reactor Inlet, °C Reactor Outlet, °C Reactor Inlet, kPa Reactor Outlet, kPa The reactor effluent temperature of ________°C is ___________ the critical temperature for some or all of the chemical components. Since each of these chemical components in the effluent stream would exist in the ________ phase as a pure compound, the mixture of these chemical compounds in the reactor effluent exists in the ________ phase too. The reactor effluent temperature of ________°C is below the critical temperature for some or none of the compounds. If none, then the above paragraph only applies; otherwise, complete the next paragraph. The boiling temperatures for pure water, pure styrene monomer, and pure ethylbenzene are 137, 197, 186°C, respectively, at 330 kPa. These values were determined using HYSYS. Since the reactor effluent temperature is _______________ than the boiling temperatures of these chemical compounds at 330 kPa, these compounds would exist in the _________ phase. 2. At what temperature in °C do two phases (vapor-liquid) start to occur on cooling? Do three phases (vapor-liquid-liquid) start to occur on cooling? In the HYSYS program, the Performance Table for Process Unit E1 provides the answers to this question for a reactor inlet temperature of _______°C. The number of data points to be used in the full table is 500. Two-phases (vapor-liquid) occur at the dew point of _______°C for a Vf = 1, while three phases (vapor-liquid-liquid) occur at around _______°C for when a heavy-liquid mass flow starts to occur. Page 1 of 2 your_name Problem SM.2 Questions date 3. On a molar basis, what fraction of Stream S12 after cooling to 38°C goes to the vapor phase of the decanter? To the organic phase? To the aqueous phase? Reactor Inlet Temperature of _______°C Fraction of S12 in vapor phase = Fraction of S12 in organic phase = Fraction of S12 in aqueous phase = nS 13 nS 12 nS 14 nS 12 nS 15 nS 12 __________ kgmol / h __________ kgmol / h __________ kgmol / h __________ kgmol / h __________ kgmol / h __________ kgmol / h ____________ ____________ ____________ 4. On a mass basis, what fraction of Stream S12 after cooling to 38°C goes to the vapor phase of the decanter? To the organic phase? To the aqueous phase? Reactor Inlet Temperature of _______°C Fraction of S12 in vapor phase = mS 13 _____________ kg / h ____________ mS 12 _____________ kg / h Fraction of S12 in organic phase = mS 14 _____________ kg / h ____________ mS 12 _____________ kg / h Fraction of S12 in aqueous phase = mS 15 _____________ kg / h ____________ mS 12 _____________ kg / h Page 2 of 2