Ch. 11 Gas Laws Chapter Review Answers Particles of matter are
advertisement

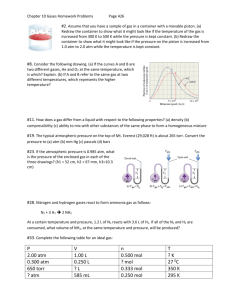
Ch. 11 Gas Laws Chapter Review Answers 1. Particles of matter are always in motion. Gas particles are small and have a huge amount of space between them compared to their size. Because they are so far apart, they have no attraction between each other, and they have highly elastic collisions. The temperature of the gas is the average kinetic energy of the gas particles. 2. An ideal gas is a theoretical gas that has perfect particle qualities that fit the molecular-kinetic theory of matter. 3. Pressure: a. The fast moving gas particles strike the sides of the container they are in. b. The same force applied to a smaller area will calculate to a greater pressure. 4. Mercury and Pressure: a. The pressure of the mercury in the tube is equal to the atmospheric pressure at sea-level, which is 101.325 kPa, or 1 atm, or 760 mmHg. b. 10.3 m, or approximately 34 feet. c. Mercury’s density is 13.5 times greater than the density of water, therefore, it exerts 13.5 times more pressure than a column of water the same size. 5. Units of Pressure: a. mmHg, in.Hg, torr, bar, psi, atm., Pa b. 1 atm = 760 mmHg c. A Pascal is the force of 1 N exerted over an area of 1 m2. 1 Pa = 1N/1 m2. d. 1 atm = 101.325 kPa, or 101,325 Pa 6. Dalton’s Law of Partial Pressures a. The partial pressures are the pressures that each individual gas exerts in the mixture of gases. b. They are independent of each other. 7. 543 mm 8. Torr units a. 950. torr b. 1.88 torr c. 3.61 × 107 torr d. 5.78 × 109 torr 9. More pressure units a. 0.164 atm b. 324,000 Pa or 3.24 × 105 Pa c. 40.4 mmHg 10. 166.190 torr 11. 699.8 torr 12. Inverse Proportion 13. The molecules are closer together to each other and the container walls, and strike a given unit area of the container of the walls of the container more frequently. 14. Direct proportion 15. Direct proportion 16. Gas particles that are at a higher temperature will have a greater kinetic energy. Therefore at a constant volume for the container, the gas particles will collide with a given unit area of the walls of the container with more force and more frequently resulting in a greater pressure. 17. P1 V1 T1 = P2 V2 T2 Or 18. Gases! a. 100.0 mL b. 1.4 mmHg 19. Calculations: a. 93.3 mL PV T = 𝓀 20. 21. 22. 23. 24. 25. 26. 27. 28. 29. 30. 31. 32. 33. 34. 35. 36. 37. 38. 39. 40. b. 588 K or 315 °C. c. 29.9 mL 121 K or −152 °C 142 mL 0.360 atm. 3.41 L 127 °C 38.8 kPa Volumes: a. 225.0 mL b. 1800. mL 605,000 mL 540 K or 267 °C 260. K or −13 °C −16 °C 243 L −43 °C 9.98 m3 / day Gas law specifics: a. Gay-Lussac’s law of combining gas volumes only works when the gases have the same temperature and pressure. b. According to Avogadro’s law, if the temperature of a gas and the pressure of the gas are the same, then volume and number of particles are directly related, more particles = more volume, less particles = less volume. Avogadro’s Law: a. The molar ratios would be the same as the volume ratios of the gaseous reactants and gaseous products, provided they were at the same temperature and pressure. b. Using volume ratios to solve stoichiometry problems is risky because all the gases must be measured at the same temperature and pressure, and will only work with gaseous chemicals. More Avogadro’s Law: a. At constant pressure and temperature, the relationship between particle number and volume is a direct proportion. b. Avogadro’s Law states that the relationship of volume of a gas to the number of particles is represented by: V = n ∙ 𝓀 V = volume, n = number of moles, and k = is the proportionality constant of volume & moles. At STP, 22.4 L of gas contains 1 mole of that gas. Therefore, the mass of 22.4 L of a gas at STP, will have a mass equal to the molar mass of that gas. Ideal Gas Law: a. The ideal gas law is most useful when you know 3 of the following 4 variables: volume, pressure, temperature, and number of moles of the gas. b. You must pay attention to the units of the variables, because of the gas constant used and its units, to ensure that units cancel and give the correct result. More Ideal Gas Law: a. PV = nRT b. The ideal gas law relates pressure, volume, number of particles, and temperature of any gas that is under conditions where it behaves like an ideal gas. Particle Calculations: a. 1.08 × 1023 molecules of H2 b. 1.08 × 1023 molecules of CO2 41. 42. 43. 44. 45. 46. 47. 48. 49. 50. 51. 52. 53. c. 2.16 × 1023 molecules of NH3 Mole Calculations: a. 1.00 mol N2 b. 0.250 mol Cl2 c. 5.58 × 10−3 mol Ne d. 3.13 × 10−3 mol NH3 Mass Calculations: a. 1.01 g H2 b. 5.50 g CO2 c. 0.0429 g SO2 d. 5.77 × 10−3 g F2 Volumes: a. 5.60 L O2 b. 2.80 L CO c. 0.0112 L H2S d. 2.96 × 108 L NH3 More Volumes: a. 37.5 L C2H2 b. 37.5 L H2O c. 93.8 L O2 More Calculations: a. 0.250 mol H2 b. 0.250 mol Cu c. 15.9 g Cu 29.0 L CO2 and 58.0 L H2O 1,50̅0 L of Air. or 1.50 × 103 L of Air. Methanol Reaction: a. The excess reactant is CO. b. 37.0 mL CO c. 413 mL CH3OH Convert to atmospheres: a. 14.2 atm b. 4.5 atm c. 0.777 atm Calculate Liters: a. 39.4 L H2 b. 14.9 L NH3 c. 3.81 L O2 Calculate Moles: a. 0.0646 mol b. 0.030 mol c. 0.0377 mol Calculate Mass: a. 15.3 g O2 b. 2.23 g NH3 c. 0.299 g SO2 Diffusion is the process where gas molecules begin in a concentrated volume, then through molecular motion and collisions, spread out to evenly occupy a larger volume. 54. If two different gases are at the same temperature, they will have the same average kinetic energy. Kinetic energy involves both velocity and mass. Larger mass must move slower and smaller mass must move faster so they will have the same energy. As a result, heavier molecules/atoms will diffuse/effuse more slowly than lighter molecules/atoms. The difference in the rates of the two gas’ effusion and diffusion is the square roots of their molar masses. 55. Since both ammonia and ethanol are at the same temperature, the ammonia will be smelled first because it will diffuse faster than ethanol because ammonia has a lighter mass than ethanol. 56. Rates of Effusion: a. 𝑅𝑎𝑡𝑒 𝐻2 𝑅𝑎𝑡𝑒 𝑁2 = b. 𝑅𝑎𝑡𝑒 𝐹2 𝑅𝑎𝑡𝑒 𝐶𝑙2 = 57. 𝑣𝑒𝑙𝑜𝑐𝑖𝑡𝑦 𝐻2 𝑣𝑒𝑙𝑜𝑐𝑖𝑡𝑦 𝑁𝑒 58. 𝑣𝑒𝑙𝑜𝑐𝑖𝑡𝑦 𝑆𝑂2 𝑣𝑒𝑙𝑜𝑐𝑖𝑡𝑦 𝐶𝑙2 59. 60. 61. 62. 63. 64. 65. 66. 67. 68. 69. 70. 71. 72. 73. 74. 75. 76. 77. 78. = = √28.02 √2.02 = 3.72 √70.90 = √38.00 √20.18 = 3.16 √2.02 1.366 √𝑀𝑜𝑙𝑎𝑟 𝑀𝑎𝑠𝑠 𝐶𝑙2 √𝑀𝑜𝑙𝑎𝑟 𝑀𝑎𝑠𝑠 𝑆𝑂2 ∶ 𝑉𝑒𝑙𝑜𝑐𝑖𝑡𝑦 𝑆𝑂2 = √70.90 √64.065 × 324 𝑚 𝑠 = 341 𝑚 𝑠 0.54 atm 2.18 L 313 mL 0.660 atm 579 °C 299 K 7940 torr 4.00 × 108 L 64 g/mol 0.839 g 97.2 L CO diffusion is 1.691 times faster than SO3. 27.9 g/mol 0.0395 mol He 3.67 L, 45.1 g/mol Applying Models: a. An inverse proportion when graphed shows a curved line. This is because as one variable increase the other variable decreases, and vice-versa. b. A direct proportion has a straight line, as a change in one variable results in the same change in the other variable. (One increases, the other increases; one decreases, the other decreases.) One of the conditions of ideal gases are that particles have no attractions to each other, therefore even if the particles were to cool and slow down they couldn’t become a liquid or solid, because they would not have the attractive forces needed to stick together to form a liquid or solid. The size of the surface area of the tube and the dish are irrelevant because the size of the gravitational weight force of the mercury at all sizes is balanced by the air pressure force from the atmosphere. When to use an equation?: a. The combined gas law should be used when the temperature, pressure and volume of the gas are known, and change, but the amount of gas (moles) is assumed to remain constant. b. The ideal gas law is used when finding an unknown quantity of a gas when 3 of the other 4 variables (temperature, volume, pressure, number of moles) are known. With Avogadro’s law, the number of particles of two gases is the same if the gases have the same conditions, of pressure, volume and temperature. Gay-Lussac’s Law of combining volumes works if the gases are under the same pressure and temperature. Since the pressure and temperature are the same, the only variable affecting the volume then is the number of particles. This would be a direct-proportion relationship, and the number of particles of each gas would determine that gas’ contribution to the whole, and adding up all parts of the mixture will result in the total volume of the gas mixture. 79. Graph Analysis: a. The temperature is greater for B. b. The average kinetic energy is greater for B. c. The average molecular velocity is greater for B. d. Cannot be determined from the information provided in the graph. e. Cannot be determined from the information provided in the graph.