06_virology_viral_quantification
advertisement

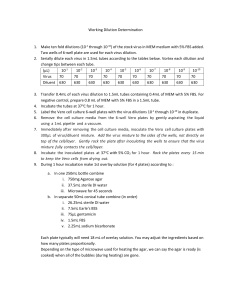
Applied Veterinary Virology: The isolation and identification of viruses using cell cultures Applied Veterinary Virology: The isolation and identification of viruses using cell cultures Authors: Prof Estelle Venter Licensed under a Creative Commons Attribution license. VIRAL QUANTIFICATION Infectivity assays for viruses Two general types of assays are based on the ability of the virus to infect cells. The first depends on quantification of individual, localized infection in a layer of cells. The plaque assay is an example of this. The second method depends on the detection of virus infection in cell cultures or animals, inoculated with serial dilutions of the virus, is better known as the end-point dilution method. Dose response curve and significance of ID50/TCID50 Figure 4: Dose response curve Examine this graph 1|Page Applied Veterinary Virology: The isolation and identification of viruses using cell cultures • of the 50% area of response a very small alteration in the host is reflected by an exaggerated difference in the percentage of the host affected • at high extremes of the curve, a much greater variation in the dose is required to affect the • number of hosts thus the point of maximum sensitivity of the assay system can be established at the 50% level Thus, the unit of measurement become a 50% dose and may be expressed in various ways, e.g. • LD5O – (lethal dose 50), • PD5O (paralytic dose 50) • TCID5O (tissue culture infected dose 50) • It is also called the endpoint method - find the endpoint dilution at which 50% of the host cells or organisms are damaged This is only a statistical unit • terms of the conditions, e.g. volume of inoculum, route of infection, host system must be defined. Example of the calculation of the 50% end point by the method of Reed & Muench (1938). Table 3: Calculation of the TCID 50 of a virus suspension DEATH / SURVIVAL Virus dilution Lab record D S (P) (N) P P+N D/S Cumulative % ± 10-1 5/5 5 0 22 0 100 10-2 5/5 5 0 17 0 100 10-3 5/5 5 0 12 0 100 10-4 4/5 4 1 7 1 88 10-5 2/5 2 3 3 4 43 10-6 1/5 1 4 1 8 11 10-7 0/5 0 5 0 13 0 50 % Proportionate distance = 2|Page % 𝑚𝑜𝑟𝑡𝑎𝑙𝑖𝑡𝑦 > 50−50 % 𝑚𝑜𝑟𝑡𝑎𝑙𝑖𝑡𝑦 > 50− % 𝑚𝑜𝑟𝑡𝑎𝑙𝑖𝑡𝑦 < 50 Applied Veterinary Virology: The isolation and identification of viruses using cell cultures = = = 88−50 88−42 38 45 0,8 (i.e. proportional distance between the 10-4 and 10-5 dilution at which 50 % of the host system is affected) By formula: LD50= log of lower dilution + (prof. distance x log. of dilution factor)= 4 + (0,8 x 1) By same calculation the original suspension before dilution must contain 63100 LD 50 (Anti log of 8 = 0,6310) per unit volume. A dilution of 10-4,8 contains 1 LD50. Plaque assay In the plaque assay method, the number of plaques produced is proportional to the concentration of the virus inoculated, that is, the dose-response curve is linear. This proportionally indicates that one virus particle is responsible for the formation of each plaque. It also indicates that one virus particle is sufficient to infect a cell. The endpoint method is still used for certain viruses that do not cause sufficient CPE’s recognized as plaques but whose presence can be detected in cultured cells, or for viruses that do not replicate in tissue culture but do cause disease in embryos or adult animals. The virus is serially diluted, and a constant volume of each dilution is inoculated into a number of similar test units (such as mice, chick embryos or cell cultures). At each dilution the proportion of infected test units (infectivity ratio) is scored e.g. • death or disease of an animal or embryo, • • degeneration of a tissue culture, or recognition of progeny virus in vitro The lower dilutions of the virus infect most the test units, and the highest dilutions infect none. The end-point is the last dilution at which virus can be detected in an inoculated culture or animal and that dilution contains at least one infective unit in the volume inoculated. The transition is not sharp, however, and only by combining the data from several dilutions is it possible to calculate the precise endpoint at which 50% of the test units are infected. At this dilution each sample contains on the average one ID50, i.e., one infectious dose for 50% of the test units. Viral titres obtained by the endpoint method are expressed in various equivalents of the ID 50: LD50 (lethal dose) if the criterion is death, PD50 (paralysis dose) if the criterion is paralysis, TC 50 or TCID50 (tissue culture dose) if the criterion is degeneration of a culture. Statistical methods according to Reed & Muench, and Kaber. 3|Page Applied Veterinary Virology: The isolation and identification of viruses using cell cultures The interpolation to obtain the ID50 can be carried out in a variety of ways. The method of Reed and Muench (1938), though not mathematically derived, yields results in fair agreement with more rigorous methods. This method is used in conjunction with a series of progressive dilutions of a virus. If the 50% endpoint lies between two dilutions, the dilution containing one ID50 is obtained by interpolation between the two dilutions that straddle the 50% value of the infectivity ratio. The interpolation assumes that in the proximity of the ID 50 the infectivity ratio varies linearly with the log dilution. The interpolated value is given by: % animals affected at dilution next above 50% - 50% % animals affected at dilution next above 50% - % animals affected at dilution below 50% This is multiplied by h, which is the log of the dilution factor employed at each step of the serial dilution of the virus. This method of calculation is only justified if one assumes that the fluctuations observed at each dilution are purely due to variations in host sensitivity to the agent under test and not to the chance presence or absence of discrete material particles. Plaque assay technique and factors which influence infective particle counts The plaque method is the fundamental assay method in virus research, and is of great value in diagnosis for it combines simplicity with accuracy and high reproducibility. It was first used with bacteriophages and these were assayed as follows: A phage-containing sample was mixed with a drop of dense liquid culture of suitable bacteria and a few millilitres of melted soft agar at 44°C. The mixture was poured over the surface of a plate or Petri-dish containing a layer of hard nutrient agar. The soft agar spreads in a thin layer and sets, and the bacteriophages diffuse through it until each meets and infects a bacterium. After 20-30 min the bacterium undergoes lysis, releasing several hundred progeny virions. These, in turn, infect neighbouring bacteria, which again lyse and infect new virus. In the meantime, the uninfected bacteria grow to form a dense, opaque lawn and after a day’s incubation the lysed areas stand out as transparent plaques against the dense background. The soft agar permits diffusion of phage to nearby cells, but prevents convection to other regions of the plate and secondary centres of infection cannot form. With animal viruses, a similar method is possible, the bacteria being replaced by a monolayer of cells growing on a solid support, and the medium is replaced by a solution containing serial dilutions of virus. Within an hour or so, most of the virions attach to cells. Soft nutrient agar or some other gelling mixture is poured over the cell layer to minimize the spread of the virus by dispersion, which would occur in liquid medium. A single virus particle infects a cell, and progeny virus produced by that virus spreads to adjacent cells. This process repeats itself and results in a localized area of infection and cell damage that can be visually identified and counted (plaques) after a period of incubation. Plaques are detected in a variety of ways: 4|Page Applied Veterinary Virology: The isolation and identification of viruses using cell cultures • The virus often kills the infected cells and produces the so-called CPE. The plaques are the detected by staining the cell layer with a dye that stains only the live cells (e.g. neutral red) or only the dead cells (trypan blue) • With certain viruses, the cells in the plaques are not killed but acquire the ability to absorb red blood cells. The plaques are revealed by haemadsorption (flooding the cell layer with a suspension of red blood cells and then washing out those not attached to infected cells) • The infected cells may fuse with neighbouring uninfected cells to form polykaryocytes which are microscopically detectable (syncytial plaques) • Often the cells of the plaque contain large amounts of viral antigens, which can be detected by immunofluorescence Each lesion is caused by a single virus particle, and the titre of the viral preparation can be directly calculated from the number of plaques and the dilution of the sample. The amount of virus quantitated by the plaque assay method is expressed as plaque-forming units (PFU). A variation of the plaque assay that is used for tumour viruses that do not cause cell death is the focus assay. A tumour virus causes cell proliferation and the formation of localized area of transformed, proliferating cells called a focus. The units are expressed as focus-forming units (FFU). Some viruses cause localized lesions on the chorioallantoic membrane of the chick embryo, which are called pocks. Each pock is formed by a single virion. 5|Page