Significant Financial Interest Review Form
advertisement

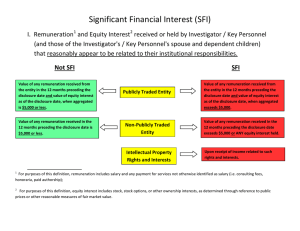
Significant Financial Interest Review Form (Form 1) Revised 01/22/15 Principal investigators (PIs) and any other individuals with responsibility for design, conduct, or reporting of a project (“Investigators”) must disclose Significant Financial Interests of themselves, their spouse, and dependent children where that financial interest may be: 1) affected by the research being conducted or, 2) in any organization that would reasonably appear to be affected by or benefit from the research being conducted. Disclosure is required at the time a new proposal is submitted (and annually thereafter if proposal is funded) and whenever there is a change in the Significant Financial Interest, as defined on page 2 under Definitions. The University of Connecticut’s Conflict of Interest policy can be found here: http://policy.uconn.edu/?p=382 Investigator Name Date Department School Phone Number E-mail address Proposal Title Amount Requested Submitted to (Sponsor) Project Start Date Project End Date If you are the Co-PI, who is the PI? If this is an Existing Grant, a Continuing Grant, or a Supplement, what is the KFS # Does this research involve human subjects? Does this research involve animal subjects? YES NO YES NO I. DISCLOSURE - SIGNIFICANT FINANCIAL INTEREST Based on the definitions of “Significant Financial Interest” from page 2, have you (“you” meaning you, your spouse, or dependent children) had in the past 12 months, currently have, or expect to have in the next 12 months, a Significant Financial Interest that reasonably appears to be related to your Institutional responsibilities or that would reasonably appear to be affected by the research for which funding is sought or are in entities whose financial interests would reasonably be affected by the research? (Note: Examples of significant financial interests include equity or ownership interests or projected annual income of an investigator, an investigator’s spouse or dependent children valued at greater than $5,000 or an equity or ownership interest of more than 5% in a single publicly traded entity or any equity ownership in a single private entity. See page two for greater detail.) YES A Significant Financial Interest may exist, and I agree to submit a Supplemental Significant Financial Interest Disclosure Form for review by the Financial Conflict of Interest in Research Committee (FCOIRC) not less than 30 days prior to the project start date, with a copy of this Review Form attached. NO A potential Significant Financial Interest does not exist at this time. I agree to submit an updated Significant Financial Interest Review Form / Supplemental Significant Financial Interest Disclosure Form, as needed, should circumstances change. II. CERTIFICATION - I certify that the above information is complete and true to the best of my knowledge and that I have read the UConn Conflict of Interest policy. I acknowledge that I am responsible for submitting updates to this information within 30 days of any change. Signature Date (Original Signature ONLY (required – a “per” signature is not acceptable) To be completed by SPS SPS # __________________ FCOI # _____________________ Page 1 of 3 Significant Financial Interest Review Form (Form 1) Revised 01/22/15 DEFINITIONS Significant Financial Interest (SFI) - PHS funding: With regard to any publicly traded entity, an SFI exists if the value of any remuneration* received from the entity in the twelve months preceding the disclosure and the value of any equity interest in the entity as of the date of disclosure, when aggregated, exceeds $5,000; or With regard to any non-publicly traded entity, an SFI exists if the value of any remuneration received from the entity in the twelve months preceding the disclosure, when aggregated, exceeds $5,000, or when the Investigator (or the Investigator’s Immediate Family) holds any equity interest (e.g., stock, stock option, or other ownership interest); or Intellectual property rights and interests (e.g., patents, copyrights), upon receipt of income related to such rights and interests. Investigators also must disclose the occurrence of any reimbursed or sponsored travel (i.e., that which is paid on behalf of the Investigator and not reimbursed to the Investigator so that the exact monetary value may not be readily available), related to their Institutional Responsibilities. Significant Financial Interest (SFI) - Non-PHS funding: An equity interest that when aggregated for the Investigator and the Investigator’s Immediate Family exceeded $5,000 over the last 12 months, and/or is expected to exceed $5,000 in value over the next 12 months as determined through reference to public prices or other reasonable measures of fair market value; or when the Investigator (or the Investigator’s Immediate Family) holds a 5% or greater equity interest (e.g., partnership, ownership, stock, stock option, or other ownership interest) in a single publicly traded entity or holds any equity interest in a non-publicly traded entity; or Salary, royalties or other payments not from the University for services (e.g., consulting fees or honoraria) that when aggregated for the Investigator and the Immediate Family over the last 12 months exceeded $5,000 or are expected to exceed $5,000 over the next 12 months. Investigators governed by FDA regulations would also have a Significant Financial Interest if one or more of the following apply: Compensation made to the investigator in which the value of compensation could be affected by the outcome of the study/research project. A proprietary interest in the tested product, including but not limited to, a patent, trademark, copyright or licensing agreement. Significant payments of other sorts, which are payments that have a cumulative monetary value of $25,000 or more made by the sponsor of a covered study to the investigator or the investigators' institution to support activities of the investigator exclusive of the costs of conducting the clinical study or other clinical studies, (e.g., a grant to fund ongoing research, compensation in the form of equipment or retainers for ongoing consultation or honoraria) during the time the clinical investigator is carrying out the study and for one year following completion of the study. The term Significant Financial Interest does not include the following types of financial interests: Salary, royalties, or other remuneration paid by the Institution to the Investigator if the Investigator is currently employed or otherwise appointed by the University, including intellectual property rights assigned to the University and agreements to share in royalties related to such rights; Income from investment vehicles, such as mutual funds and retirement accounts, as long as the Investigator does not directly control the investment decisions made in these vehicles; Page 2 of 3 Significant Financial Interest Review Form (Form 1) Revised 01/22/15 Income from seminars, lectures, or teaching engagements sponsored by a federal, state, or local government agency, an institution of higher education as defined at 20 U.S.C. 1001(a), an academic teaching hospital, a medical center, or a research institute that is affiliated with an Institution of higher education; or Income from service on advisory committees or review panels for a federal, state, or local government agency, an Institution of higher education as defined at 20 U.S.C. 1001(a), an academic teaching hospital, a medical center, or a research institute that is affiliated with an institution of higher education. Travel that is reimbursed or sponsored by a federal, state, or local government agency, an Institution of higher education as defined at 20 U.S.C. 1001(a), an academic teaching hospital, a medical center, or a research institute that is affiliated with an Institution of higher education. *Remuneration includes salary and any payment for services not otherwise identified as salary (e.g., consulting fees, honoraria, paid authorship); equity interest includes any stock, stock option, or other ownership interest, as determined through reference to public prices or other reasonable measures of fair market value Page 3 of 3