3-6 WKST
advertisement

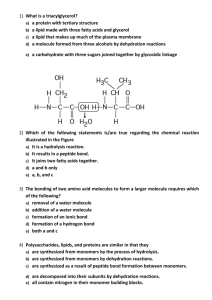
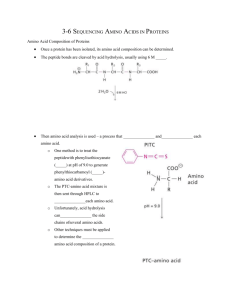
3-6 SEQUENCING AMINO ACIDS IN PROTEINS 1. Show the cleaving of the peptide bonds in Met-Phe-Ser using 6 M HCl. 2. What chemical is used to separate and quantify each amino acid? 3. Show the mechanism for treating cysteine with PITC. 4. What technique separates each amino acid after being treated with PITC? 5. What problems arise from cleaving the peptide bonds by acid hydrolysis? 6. What procedure is used to determine the sequence of amino acid residues? In general, how does it work? 7. Show how PITC can be used to remove Ala from Ala-Tyr-Asp. 8. What linkage may exist between cystine residues that must be cleaved for Edman degradation? Draw an example. Draw the structure of a compound that can cleave this linkage, and draw its structure after cleavage. What is the resulting mixture treated with that prevents the re-formation of disulfide bonds? Draw the mechanism for how it attaches to the cysteine residue. 9. Why would proteins be treated with chemicals such as proteases, cyanogens bromide, trypsin, S. aureus V8 protease, and chymotrypsin? 10. Who was the first to determine the complete sequence of a protein? What protein, when, and what did he earn for is efforts? Why did he win a second one of these?