Answer
advertisement
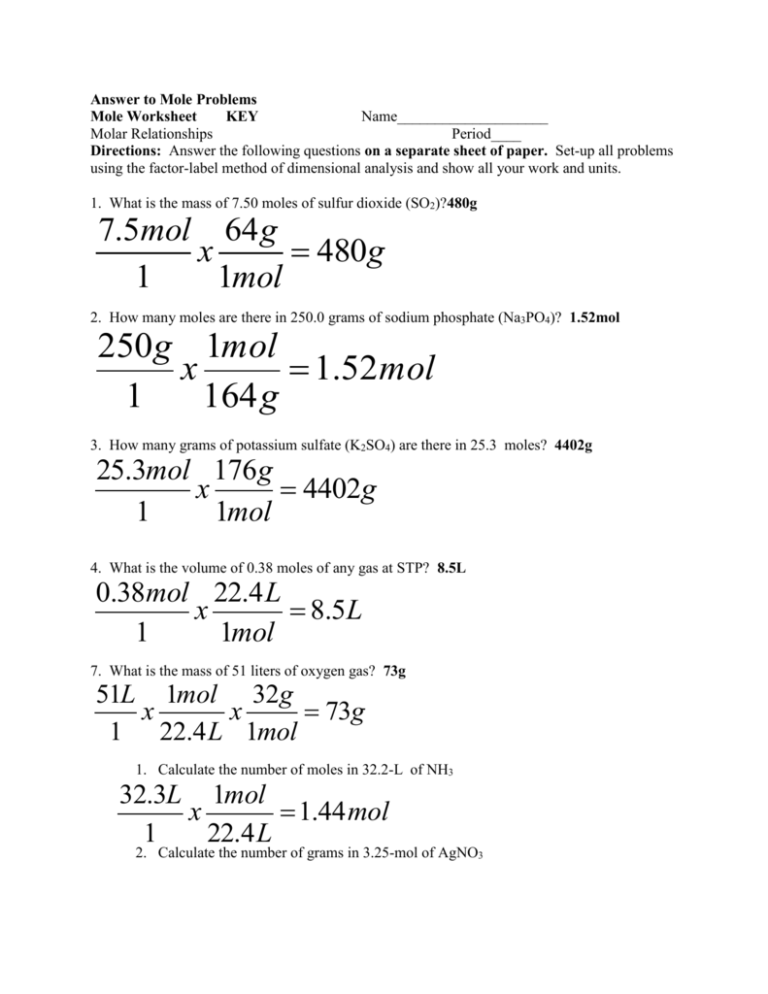
Answer to Mole Problems Mole Worksheet KEY Name____________________ Molar Relationships Period____ Directions: Answer the following questions on a separate sheet of paper. Set-up all problems using the factor-label method of dimensional analysis and show all your work and units. 1. What is the mass of 7.50 moles of sulfur dioxide (SO2)?480g 7.5mol 64g x 480g 1 1mol 2. How many moles are there in 250.0 grams of sodium phosphate (Na3PO4)? 1.52mol 250g 1mol x 1.52mol 1 164g 3. How many grams of potassium sulfate (K2SO4) are there in 25.3 moles? 4402g 25.3mol 176g x 4402g 1 1mol 4. What is the volume of 0.38 moles of any gas at STP? 8.5L 0.38mol 22.4L x 8.5L 1 1mol 7. What is the mass of 51 liters of oxygen gas? 73g 51L 1mol 32g x x 73g 1 22.4L 1mol 1. Calculate the number of moles in 32.2-L of NH3 32.3L 1mol x 1.44mol 1 22.4L 2. Calculate the number of grams in 3.25-mol of AgNO3 MM 107 14 3x16 169g 1mol 3.25mol 169g x 549g 1 1mol 8. Calculate the number of liters in 3.25-g of NH3 17g 1mol 3.26g 1mol 22.4L x x 4.30L 1 17g 1mol MM 14 3 9. Calculate the number of grams in 3.54-L of CO2 44g MM 12 16x2 1mol 3.54L 1mol 44g x x 6.95g 1 22.4L 1mol Part II 1. Calculate the % composition of Li2O. Li2O :: MM 7x2 16 30g /mol 14 Li x100 46.7% 30 16 O x100 53.3% 30 2. What is the percentage composition of a carbon-oxygen compound, given that a 95.2 g sample of the compound contains 40.8 g of carbon and 54.4 g of oxygen? 40.8 x100 42.9% 95.2 54.4 O x100 57.1% 95.2 C Part E: Empirical and Molecular Formulas 1. Determine the empirical formula of a compound with 72.4% Fe and 27.6% Oxygen. 72.4gFe 1molFe x 1.29molFe 1 56gFe 27.6gO 1molO O: x 1.725molO 1 16gO 1.29molFe 1x3 3 1.29mol 1.725molO 1.33x3 4 1.29mol Fe3O4 Fe : +6 2. Determine the empirical formula of a compound with 52.8% Sn, 12.4% Fe, 16% C and 18.8% N. 52.8gSc 1molSc 0.444molSn x 2 1 119gSc 0.221 12.4gFe 1molFe 0.221molFe Fe : x 1 1 56gFe 0.221 16gC 1molC 1.33molC C: x 6 1 12gC 0.221 18.8gC 1molN 1.34molN N: x 6 1 14gN 0.221 Sn : +8 Sn 2 FeC6 N 6 4. Determine the molecular formula for a compound that contains 12.2-g Nitrogen, 27.8-g Oxygen, and a molecular mass of 92.0 g/mol. 12.2gN 1molN 0.87molN x 0.87molN :: 1 1 14gN 0.87mol 27.8gO 1molO 1.74molO O: x 1.74molO :: 2 1 16gO 0.87mol EF NO2 :: EM 46 :: MM 92 Factor 2 N: +8 NO2 x2 N 2O4 5. Determine the molecular formula for a compound that contains 94.1% oxygen and 5.9% hydrogen and a molecular mass of 34 g/mol. 5.9gH 1molH 5.9molN H: x 5.9molH :: 1 1 1gH 5.9mol 94.1O 1molO 5.9molO O: x 5.9molO :: 1 1 16gO 5.9mol EF HO :: EM 17 :: MM 34 Factor 2 +8 HOx 2 H 2O2 6. A sample of TNT, a common explosive is analyzed and found to contain 1.03-g of nitrogen, 0.220-g hydrogen, and 1.76-g of carbon. The molar mass is 123 g/mol. What is the molecular formula? 1.03 g 1mol N x 0.0736mol / 0.0736mol 1 1 14 g 0.220 g 1mol H x 0.220mol / 0.0736mol 2.99 1 1g 1.76 g 1mol C x 0.147mol / 0.0736mol 2 1 12 g NH 3C 2 MM 123 : EM 41(multiple3) MF N 3 H 9C6 8. Azobenzene is an important intermediate in the manufacture of dyes. It contains 79.1% carbon, 5.95% hydrogen, and 15.4% nitrogen. It has a molar mass of 182g/mol. What is the molecular formula? 79.1g 1mol C x 6.59mol / 1.1mol 6 1 12 g 5.95 g 1mol H x 5.95mol / 1.1mol 5 1 1g 15.4 g 1mol N x 1.1mol / 1.1mol 1 1 14 g C6 H 5 N MM 182 : EM 91(multiple 2) MF C12 H 10 N 2