I2CNER Institute Interest Seminar Series July 25, 2012
advertisement
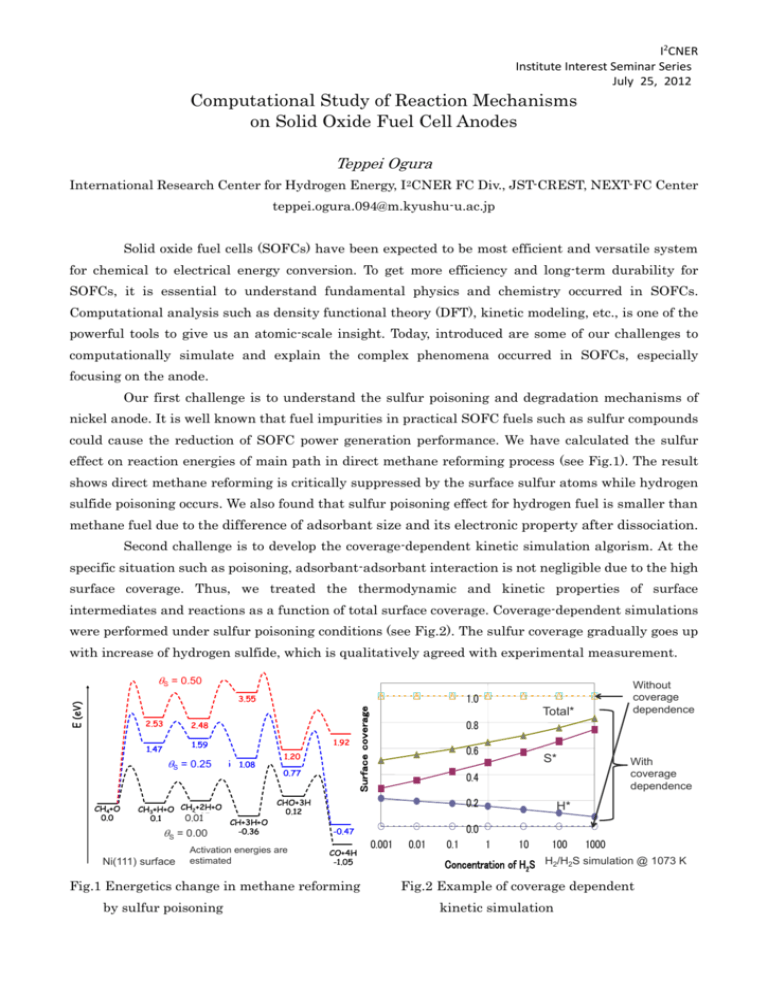
I2CNER Institute Interest Seminar Series July 25, 2012 Computational Study of Reaction Mechanisms on Solid Oxide Fuel Cell Anodes Teppei Ogura International Research Center for Hydrogen Energy, I2CNER FC Div., JST-CREST, NEXT-FC Center teppei.ogura.094@m.kyushu-u.ac.jp Solid oxide fuel cells (SOFCs) have been expected to be most efficient and versatile system for chemical to electrical energy conversion. To get more efficiency and long-term durability for SOFCs, it is essential to understand fundamental physics and chemistry occurred in SOFCs. Computational analysis such as density functional theory (DFT), kinetic modeling, etc., is one of the powerful tools to give us an atomic-scale insight. Today, introduced are some of our challenges to computationally simulate and explain the complex phenomena occurred in SOFCs, especially focusing on the anode. Our first challenge is to understand the sulfur poisoning and degradation mechanisms of nickel anode. It is well known that fuel impurities in practical SOFC fuels such as sulfur compounds could cause the reduction of SOFC power generation performance. We have calculated the sulfur effect on reaction energies of main path in direct methane reforming process (see Fig.1). The result shows direct methane reforming is critically suppressed by the surface sulfur atoms while hydrogen sulfide poisoning occurs. We also found that sulfur poisoning effect for hydrogen fuel is smaller than methane fuel due to the difference of adsorbant size and its electronic property after dissociation. Second challenge is to develop the coverage-dependent kinetic simulation algorism. At the specific situation such as poisoning, adsorbant-adsorbant interaction is not negligible due to the high surface coverage. Thus, we treated the thermodynamic and kinetic properties of surface intermediates and reactions as a function of total surface coverage. Coverage-dependent simulations were performed under sulfur poisoning conditions (see Fig.2). The sulfur coverage gradually goes up with increase of hydrogen sulfide, which is qualitatively agreed with experimental measurement. qS = 0.50 硫黄被覆率0.5 2.48 硫黄被覆率 qS = 0.250.25 1.08 CH4+O 0.0 1.92 1.59 1.47 CH3+H+O CH2+2H+O 0.05 0.1 0.01 CH+3H+O -0.36 q = 0.000.0 硫黄被覆率 1.20 0.77 Activation energies are estimated 0.6 S* H* 0.0 -0.47 CO+4H -1.05 With coverage dependence 0.4 0.2 Fig.1 Energetics change in methane reforming by sulfur poisoning Total* 0.8 CHO+3H 0.12 S Ni(111) surface Surface coverage E (eV) 2.53 Without coverage dependence 1.0 3.55 0.001 0.01 0.1 1 10 100 1000 Concentration of H2S H2/H2S simulation @ 1073 K Fig.2 Example of coverage dependent kinetic simulation