Spectroscopy Worksheet - WSU OSA-SPIE
advertisement

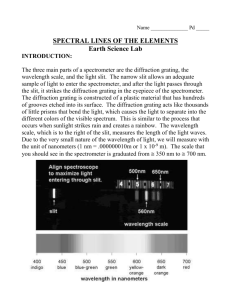
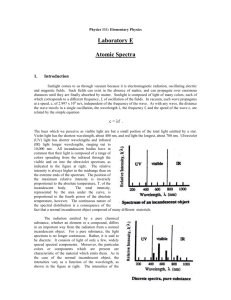
Light is a Particle! Introduction to Spectroscopy Research Question What can we learn about the microscopic structure of materials from the light they give off? Guidelines We will be constructing homemade spectrometers to investigate the light given off by a variety of sources including fluorescent, gas discharge and tungsten lamps. Introduction: Group Discussion Light can be thought of as a ray which passes straight through a lense or bounces off of a mirror, as an electromagnetic wave which oscillates through space, or even as a single particle of energy of a specific color. This single particle is called a photon. ● Atoms are the smallest building blocks which make up all things, all matter. ● Atoms are made up of a heavy, positively charged nucleus and light, negatively charged electrons orbiting around the nucleus. ● Electrons absorb light in order to stretch farther away from the nucleus and emit light when they fall back down toward the nucleus. ● The light emitted by atoms tells us how electrons move around the nucleus. Normal light is actually a mixture of a bunch of colors of light, where white light is all the colors within the visible range. Rainbows, prisms and diffraction gratings split light into each of its individual colors, showing its spectrum. By looking at the spectrum of light coming off of a source, we can directly observe qualities about the atom we could never see directly. In astronomy, the light we observe through our telescopes is all we have to work with in studying stars, galaxies, nebula, and planets. Everything we know about astronomy has come from the spectra of these objects. Content by: Washington State University OSA-SPIE Student Chapter Department of Physics and Astronomy wsu.osahost.org Equipment: Pre-cut poster board pieces (6 total) Diffraction grating square (1 in square, 500 lines/mm) Paper diffraction scale (printed) Clear and duct tape Aluminum foil pieces Constructing your spectrometer: The eyepiece of the spectrometer will have the diffraction grating in it, the spectrometer itself is just a box with a narrow slit of light allowed in so that the spectrum can be seen. 1. Construct the eyepiece a. Take the diffraction grating square and determine which way the line gets spread out by holding it up to the light. b. Square the diffraction grating with the eyepiece and mark the diffraction direction. c. Tape the diffraction grating over the eyepiece hole. 2. Construct the slit and wavelength scale a. Tape two straight edges of aluminum foil to the slit opening allowing only a narrow and straight slit of light through. b. Tape the wavelength scale so that the light slit is exactly 8.2 cm away from the first scale line, 400 nm. 3. Assemble the spectrometer pieces, duct tape into place. Never point spectrometer directly at the sun or at a laser source! Observations Observe each of the light sources and record your findings. Sketch the spectrum of each of the following sources, wavelength in nanometers. Content by: Washington State University OSA-SPIE Student Chapter Department of Physics and Astronomy wsu.osahost.org 1. Hydrogen 2. Helium 3. Neon 4. Tungsten lightbulb 5. Fluorescent lightbulb Content by: Washington State University OSA-SPIE Student Chapter Department of Physics and Astronomy wsu.osahost.org 6. Laser (Reflected off of white paper, do not look directly at the laser!) 7. Sun (Clouds or sunlight reflecting off of white paper, do not look directly at the sun!) Conclusions 1. What kind of spectra did you observe from atom gas sources? What does this tell us about the motion of electrons in an atom? 2. If you saw a cloud of glowing gas in your telescope, how might you tell what kinds of atoms make up that cloud? 3. What kind of spectrum does laser light have? 4. How is the spectrum from a tungsten filament light bulb different from the atom gas sources? 5. If you look closely at the spectrum of sunlight, you will notice dark lines where the full rainbow is dimmed. Which of the above gas(es) do many of these lines correspond to? Content by: Washington State University OSA-SPIE Student Chapter Department of Physics and Astronomy wsu.osahost.org