VO2HydroSynthesisOptimization
advertisement
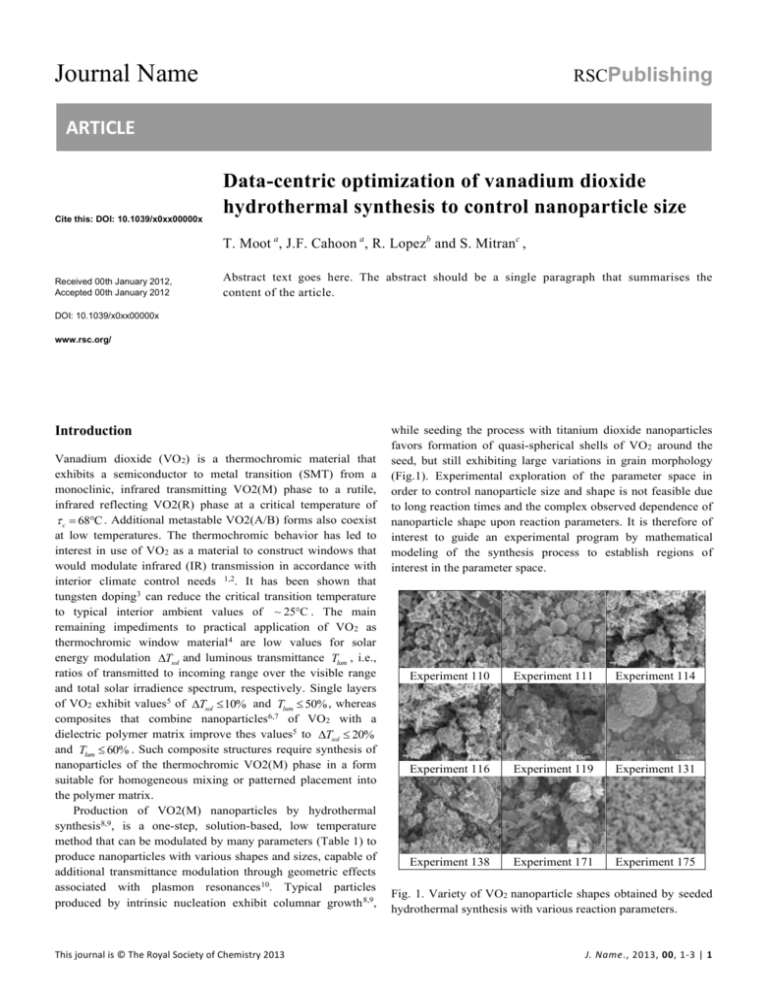
Journal Name RSCPublishing ARTICLE Cite this: DOI: 10.1039/x0xx00000x Data-centric optimization of vanadium dioxide hydrothermal synthesis to control nanoparticle size T. Moot a, J.F. Cahoon a, R. Lopezb and S. Mitranc , Received 00th January 2012, Accepted 00th January 2012 Abstract text goes here. The abstract should be a single paragraph that summarises the content of the article. DOI: 10.1039/x0xx00000x www.rsc.org/ Introduction Vanadium dioxide (VO 2) is a thermochromic material that exhibits a semiconductor to metal transition (SMT) from a monoclinic, infrared transmitting VO2(M) phase to a rutile, infrared reflecting VO2(R) phase at a critical temperature of c 68C . Additional metastable VO2(A/B) forms also coexist at low temperatures. The thermochromic behavior has led to interest in use of VO2 as a material to construct windows that would modulate infrared (IR) transmission in accordance with interior climate control needs 1,2. It has been shown that tungsten doping3 can reduce the critical transition temperature to typical interior ambient values of ~ 25C . The main remaining impediments to practical application of VO 2 as thermochromic window material 4 are low values for solar energy modulation Tsol and luminous transmittance Tlum , i.e., ratios of transmitted to incoming range over the visible range and total solar irradience spectrum, respectively. Single layers of VO2 exhibit values5 of Tsol 10% and Tlum 50% , whereas composites that combine nanoparticles6,7 of VO2 with a dielectric polymer matrix improve thes values5 to Tsol 20% and Tlum 60% . Such composite structures require synthesis of nanoparticles of the thermochromic VO2(M) phase in a form suitable for homogeneous mixing or patterned placement into the polymer matrix. Production of VO2(M) nanoparticles by hydrothermal synthesis8,9, is a one-step, solution-based, low temperature method that can be modulated by many parameters (Table 1) to produce nanoparticles with various shapes and sizes, capable of additional transmittance modulation through geometric effects associated with plasmon resonances 10. Typical particles produced by intrinsic nucleation exhibit columnar growth 8,9, This journal is © The Royal Society of Chemistry 2013 while seeding the process with titanium dioxide nanoparticles favors formation of quasi-spherical shells of VO 2 around the seed, but still exhibiting large variations in grain morphology (Fig.1). Experimental exploration of the parameter space in order to control nanoparticle size and shape is not feasible due to long reaction times and the complex observed dependence of nanoparticle shape upon reaction parameters. It is therefore of interest to guide an experimental program by mathematical modeling of the synthesis process to establish regions of interest in the parameter space. Experiment 110 Experiment 111 Experiment 114 Experiment 116 Experiment 119 Experiment 131 Experiment 138 Experiment 171 Experiment 175 Fig. 1. Variety of VO2 nanoparticle shapes obtained by seeded hydrothermal synthesis with various reaction parameters. J. Name., 2013, 00, 1-3 | 1 ARTICLE Experiments and methods Materials Vanadium Pentoxide (98+%), Oxalic Acid Dihydrate (99.5+%), and Titanium Dioxide (aeroxide P25), and acetonitrile were bought from Fisher Scientific and used without further purification. Synthesis Varying amounts of vanadium pentoxide (0.0417 g – 0.1265 g), oxalic acid dihydrate (0.0726 g – 1.9376 g), acetonitrile (0 g – 24.5532 g) and titanium dioxide (0 g – 0.0468 g) were added to deionized water (14.108 g – 43.9753 g) totaling a solution ranging from 37.9474 mL to 47.7108 mL and placed directly into a 50 mL teflon-lined stainless steel sealed autoclave. The autoclave was placed into a box furnace and heated to 210 C – 300 C for 3 to 84 hours then allowed to cool down to room temperature before being opened. The precipitate was collected by centrifuge and washed with nanopure water and ethanol and typically dried at ~100 C for ~2 hours. The set of hydrothermal synthesis parameters is listed in Table 1. Based upon an empirical understanding of the influence of reaction parameters upon nanoparticle size a series of 96 reactions were carried out as specified in Table 2. Journal Name 𝐹𝐴𝑑𝐻𝑜𝑐,𝑚𝑎𝑥 = 0.064. Log-normal distributions were used to describe grain size, and parameter sensitivity was obtained by correlation analysis (Table 1). Quadratic optimization of the extracted PDFs furnished parameter values (amount of TiO 2 precursor ~0.03g, reaction time at high temperature of ~9h), and predicted an estimated maximum value of 𝐹𝑚𝑎𝑥,𝑒𝑠𝑡 = 0.12. We recently carried out the experiments with parameters suggested by the mathematical model, and obtained an even better performance figure of 𝐹𝑚𝑎𝑥,𝑒𝑠𝑡 = 0.27, a 422% increase over 𝐹𝐴𝑑𝐻𝑜𝑐,𝑚𝑎𝑥 .. Results and Discussion B Headings should always be subordinate to A headings e.g. Synthetic procedures, Materials and methods, Crystallography. C headings should always be subordinate to B headings e.g. General procedure for synthesis of compound X. The main paragraph text follows directly on here. The main text of the article should go here with headings as appropriate. Inserting Graphics Graphics should be inserted where they are first mentioned (unless they are equations, which appear in the flow of the text). They can be single column or double column as appropriate. Characterization Powder X-ray diffraction (XRD) experiments were performed on a Rigaku Multiflex from 20 to 60 2 at a scan rate of 2 2/min. Ratios of VO 2 (M): VO2 (B) were taken from the relative peak heights at 27.8 2 and 25.5 2, respectively. Scanning electron microscopy (SEM) images were obtained using a Hitachi S-4700 Cold Cathode Field Emission Scanning Electron Microscope with powder samples deposited on carbon tape. Energy dispersion spectroscopy (EDS) was taken with an INCA PentaFET -x3 (Oxford instruments) attached to the SEM and multiple values over a 5+ micron area were averaged. Differential scanning calorimetry (DSC) was done on a TA Instruments DSC Q200 with a liquid N2 cooling unit from 0C to 100C at a scanning rate of 10C/min . Table 3 lists the Synthesis parameter optimization Our goal was to maximize the objective function 𝐹(𝑝) = 𝑃𝑉𝑂2 (𝑀) (1 − 𝑟)3 , where 𝑃𝑉𝑂2 (𝑀) is the percentage of VO 2(M) phase determined by XRD, 𝑝 is a vector of experimental processing parameters, and 𝑟 = 𝑅𝑇𝑖𝑂2 /𝑅𝑔𝑟𝑎𝑖𝑛 is the ratio of the TiO2 precursor grain size to that of the synthesized grain, assuming a spherical shell model. The objective function expresses the desire to obtain small grain sizes with high VO2(M) content, and the highest value obtained in the ad hoc experimental investigation of the parameter space was 2 | J. Name., 2012, 00, 1-3 Conclusions The conclusions section should come at the end of article, before the acknowledgements. Acknowledgements The acknowledgements come at the end of an article after the conclusions and before the notes and references. Notes and references a Department of Chemistry, University of North Carolina, Chapel Hill, NC 27599-3290. b Department of Physics and Astronomy, University of North Carolina, Chapel Hill, NC 27599-3255. c Department of Chemistry, University of North Carolina, Chapel Hill, NC 27599-3250, Email: mitran@unc.edu. † Footnotes should appear here. These might include comments relevant to but not central to the matter under discussion, limited experimental and spectral data, and crystallographic data. This journal is © The Royal Society of Chemistry 2012 Journal Name ARTICLE Electronic Supplementary Information (ESI) available: [details of any supplementary information available should be included here]. See DOI: 10.1039/b000000x/ 1 C. G. Granqvist, Thin Solid Films, 1990, 193-194, 730–741. 2 T. D. Manning, I. P. Parkin, M. E. Pemble, D. Sheel and D. Vernardou, Chem. Mater., 2004, 16, 744–749. 3 M. Tazawa, P. Jin and S. Tanemura, Appl. Opt., 1998, 37, 1858– 1861. 4 S. Y. Li, G. A. Niklasson and C. G. Granqvist, in Thin Solid Films, 2012, vol. 520, pp. 3823–3828. 5 Y. Gao, H. Luob, Z. Zhanga, L. Kang, Z. Chena, J. Dua, M. Kanehiraa and C. Cao, Nano Energy, 2012, 1, 221–246. 6 S. Y. Li, G. A. Niklasson and C. G. Granqvist, J. Appl. Phys., 2010, 108. 7 S.-Y. Li, G. A. Niklasson and C. G. Granqvist, J. Appl. Phys., 2011, 109, 113515. 8 C. Cao, Y. Gao and H. Luo, J. Phys. Chem. C, 2008, 112, 18810– 18814. 9 Z. Zhang, Y. Gao, Z. Chen, J. Du, C. Cao, L. Kang and H. Luo, Langmuir, 2010, 26, 10738–10744. 10 R. Lopez, T. E. Haynes, L. A. Boatner, L. C. Feldman and R. F. Haglund, Opt. Lett., 2002, 27, 1327–1329. This journal is © The Royal Society of Chemistry 2012 J. Name., 2012, 00, 1-3 | 3